

亚硝基-烯(Nitroso-ene)反应的研究进展
收稿日期: 2012-07-18
修回日期: 2012-08-08
网络出版日期: 2012-08-10
基金资助
上海高校青年教师培养资助计划(No. yyy11015)资助项目.
Research Progress on Nitroso-ene Reaction
Received date: 2012-07-18
Revised date: 2012-08-08
Online published: 2012-08-10
Supported by
Project supported by the Young Teachers Program of Universities in Shanghai (No. yyy11015).
烯丙基胺类化合物是有机化学中的重要合成中间体, 亚硝基-烯(nitroso-ene)反应是合成这类化合物最为高效的方法之一. 在一些天然产物和药物分子的全合成中, 亚硝基-烯反应也往往作为关键步骤. 这篇综述主要介绍了亚硝 基–烯反应的发展、应用以及机理研究方面的进展.
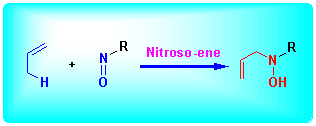
关键词: 烯丙基胺; 亚硝基化合物; 亚硝基-烯(nitroso-ene)反应; 天然产物合成
黄莎华 , 霍华兴 , 李文华 , 洪然 . 亚硝基-烯(Nitroso-ene)反应的研究进展[J]. 有机化学, 2012 , 32(10) : 1776 -1791 . DOI: 10.6023/cjoc201207026
Nitroso-ene reaction is one of the most efficient methods to construct allylamines which are the versatile building block in synthetic organic chemistry. The reaction has been embedded as the key step in the total synthesis of natural products and pharmaceuticals. This review focuses on the development, application and mechanistic study of nitroso-ene reaction.

[1] (a) Meyer, V.; Locher, J. Ber. 1874, 7, 670.
(b) Meyer, V.; Locher, J. Ber. 1874, 7, 1506.
(c) Baeyer, A.; Caro, H. Ber. 1874, 7, 809.
(d) Baeyer, A. Ber. 1874, 7, 1638.
(e) Baeyer, A.; Caro, H. Ber. 1874, 7, 963.
[2] Gowenlock, B. G.; Richter-Addo, G. B. J. Chem. Educ. 2008, 85, 1243
[3] Lee, M. D.; Dunne, T. S.; Siegel, M. M.; Chang, D. D.; Morton, G. O.; Borders, D. B. J. Am. Chem. Soc. 1987, 109, 3464.
[4] Kersten, R. D.; Dorrestein, P. C. Nat. Chem. Biol. 2010, 6, 636
[5] Boyer, J. H. In The Chemistry of the Nitro and Nitroso Groups, Part 1, Ed.: Patai, S., Interscience Publishers, New York, 1969, Chapter 5.
[6] Zuman, P.; Shah, B. Chem. Rev. 1994, 94, 1621.
[7] (a) Wichterle, O. Collect. Czech. Chem. Commun. 1947, 12, 292.
(b) Arbuzov, Y. A. Dokl. Akad. Nauk SSSR 1948, 60, 993.
[8] Lewis, J. W.; Myers, P. L.; Ormerod, J. A. J. Chem. Soc., Perkin Trans. 1 1972, 2521.
[9] Banks, R. E.; Barlow M. G.; Haszeldine, R. N. J. Chem. Soc. 1965, 4714.
[10] For reviews, see: (a) Ager, D. J.; Prakash, I.; Schaad, D. R. Chem. Rev. 1996, 96, 835.
(b) Senanayake, C. H. Aldrichim. Acta 1998, 31, 3.
(c) Lait, S. M.; Rankic, D. A.; Keay, B. A. Chem. Rev. 2007, 107, 767.
[11] (a) Oppolzer, W.; Tamura, O. Tetrahedron Lett. 1990, 31, 991.
(b) Oppolzer, W.; Tamura, O.; Sundarababu, G.; Signer, M. J. Am. Chem. Soc. 1992, 114, 5900.
(c) Momiyama, N.; Yamamoto, H. Org. Lett. 2002, 4, 3579.
[12] Goelitz, P.; Meijere, A. Angew. Chem. 1977, 89, 892.
[13] (a) Aston, A.; Menard, M. J. Am. Chem. Soc. 1935, 57, 1922.
(b) Forrester, A. R.; Hepburn, S. P. J. Chem. Soc. C 1971, 3322.
(c) Goldman, J. Tetrahedron 1973, 29, 3833.
[14] Druellinger, M. L. J. Heterocycl. Chem. 1976, 13, 1001.
[15] (a) Adam, W.; Bottle, S. E.; Peters, K. Tetrahedron Lett. 1991, 32, 4283.
(b) Torssell, K. Tetrahedron 1970, 26, 2759.
(c) Forrester, A. R.; Henderson, J.; Reid, K. Tetrahedron Lett. 1983, 24, 5547.
[16] (a) Ginsburg, V. A. J. Org. Chem. USSR (Engl. Trans.) 1974, 10, 1427.
(b) Barr, A.; Hazeldine, R. N. J. Chem. Soc. 1955, 1881.
[17] (a) Lin, C.-T.; Hsu, W.-J. Can. J. Chem. 1989, 67, 2153.
(b) Viehe, H. G.; Merenyi, R.; Francotte, E.; Van Meerssche, M.; Germain, G.; Declercq, J. P.; Bodart-Gilmont, J. J. Am. Chem. Soc. 1977, 99, 2340.
(c) Gouverneur, V.; Dive, G.; Ghosez, L. Tetrahedron: Asymmetry 1991, 12, 1173.
(d) Christie, C. C.; Kirby, G. W.; McGuian, H.; Mackinnon, J. W. M. J. Chem. Soc., Perkin. Trans. 1 1985, 1972.
[18] (a) Knight, G. T. J. Chem. Soc., Chem. Commun. 1970, 1016.
(b) Johannsen, M.; Jùrgensen, K. A. Chem. Rev. 1998, 98, 1689.
[19] (a) Glaser, R.; Murmann, R. K.; Barnes, C. L. J. Org. Chem. 1996, 61, 1047.
b) Stowell, J. C. J. Org. Chem. 1971, 36, 3055.
(c) Yamamoto, H.; Momiyama, N. J. Chem. Soc., Chem. Commun. 2005, 3514.
[20] (a) Miller, M. J.; Bodnar, B. S. Angew. Chem., Int. Ed. 2011, 50, 5630.
(b) Janey, J. M. Angew. Chem., Int. Ed. 2005, 44, 4292.
(c) Merino, P.; Tejero, T. Angew. Chem., Int. Ed. 2004, 43, 2995.
(d) Studer, A. Synthesis 1996, 793.
(e) Voget, P. F.; Miller, M. J. Tetrahedron 1998, 54, 1323.
[21] Weinreb, S. M.; Staib, R. R. Tetrahedron 1982, 38, 3113.
[22] (a) Yamamoto, H.; Kawasaki, M. Bull. Chem. Soc. Jpn. 2007, 80, 595.
(b) Oppolzer, W.; Tamura, O.; Deerberg, J. Helv. Chim. Acta 1992, 75, 1965.
[23] Guo, H.; Cheng, L.; Cun, L. F.; Gong, L. Z.; Miac, A. G.; Jiang, Y. Z. J. Chem. Soc., Chem. Commun. 2006, 429.
[24] (a) Brown, S. P.; Brochu, M. P.; Sinz C. J.; MacMillan, D. W. C. J. Am. Chem. Soc. 2003, 125, 10808.
(b) Yanagisawa, A.; Arai, T. Chem. Commun. 2008, 1165.
[25] Mikami, K.; Shimizsu, M. Chem. Rev. 1992, 92, 1021.
[26] Alder, K.; Pascher, F.; Schimitz, A. Dtsch. Chem. Ges. 1943, 76, 27.
[27] (a) Magnus, P.; Lacour, J.; Goldham, I.; Mugrage, B.; Bauta, W. B. Tetrahedron 1995, 51, 11087.
(b) Seebach, D.; Overhand, M.; Kuèhnle, F. N. M.; Martinoni, B.; Oberer, L.; Hommel, U.; Widmer, H. Helv. Chim. Acta 1996, 79, 913.
(c) Cole, D. C. Tetrahedron 1994, 50, 9517.
(d) Abele, S.; Seebach, D. Eur. J. Org. Chem. 2000, 1.
(e) Burgess, K.; Liu, L.; Pal, B. J. Org. Chem. 1993, 58, 4758.
(f) Trost, B. M.; Van Vranken, D. L. J. Am. Chem. Soc. 1993, 115, 444.
[28] Banks, R. E.; Barlow, M. G.; Haszeldine, R. N. J. Chem. Soc. 1965, 4714.
[29] Keussler, V.; Luttke, W. Z. Elektrochem. 1959, 63, 614
[30] (a) Corrie, J. E. T.; Kirby, G. W.; Mackinnon, J. W. M. J. Chem. Soc., Perkin Trans. 1 1985, 883.
(b) Quadrelli, P.; Mella, M.; Invernizzi, A. G.; Caramella, P. Tetrahedron 1999, 55, 10497.
(c) Quadrelli, P.; Campari, P.; Mella, M.; Caramella, P. Tetrahedron Lett. 2000, 41, 2019.
[31] Quadrelli, P.; Invernizzi, A. G.; Caramella, P. Tetrahedron Lett. 1996, 37, 1909.
[32] (a) Knight, G. T.; Pepper, B. Tetrahedron 1971, 27, 6201.
(b) Knight, G. T.; Loadman, M. J. R. J. Chem. Soc., B 1971, 2107.
[33] Adam, W.; Krebs, O. Chem. Rev. 2003, 103, 4131.
[34] (a) Corrie, J. E. T.; Kirby, G. W.; Mackinnon, J. W. M. J. Chem. Soc., Perkin Trans. 1 1985, 883.
(b) Kirby, G. W.; McGuigan, H.; McLean, D. J. J. Chem. Soc., Perkin Trans. 1 1985, 1961.
[35] Kirby, G. W. Chem. Soc. Rev. 1977, 6, 12.
[36] Keck, G. E.; Webb, R. R.; Yates, J. B. Tetrahedron 1981, 37, 4007.
[37] Ensley, H. E.; Mahadevan, S. Tetrahedron Lett. 1989, 30, 3255.
[38] (a) Schollkopf, U; Tonne, P.; Schafer, H.; Markusch, P. Ann. Chem. 1969, 722, 45.
(b) Schollkopf, U; Tonne, P. Ann. Chem. 1971, 753, 135.
[39] (a) O’Bannon, P. E.; Dailey, W. P. Tetrahedron Lett. 1988, 29, 987.
(b) O’Bannon, P. E.; Dailey, W. P. Tetrahedron Lett. 1988, 29, 5719.
[40] Quadrelli, P.; Mella, M.; Caramella, P. Tetrahedron Lett. 1998, 39, 3233.
[41] Quadrelli, P.; Mella, M.; Invernizzi, A. G.; Caramella, P. Tetrahedron 1999, 55, 10497.
[42] Adam, W.; Bottke, N.; Krebs, O.; Saha-Moèller, C. R. Eur. J. Org. Chem. 1999, 1963.
[43] Iwasa, S.; Fakhruddin, A.; Tajima, K.; Nishiyama, H. Tetrahedron Lett. 2004, 45, 9323.
[44] Malkov, A. V. Atkinson, D.; Kabeshov, M. A.; Edgar, M. Adv. Synth. Catal. 2011, 353, 3347.
[45] Read de Alaniz, J.; Frazier, C. P.; Engelking, J. R. J. Am. Chem. Soc. 2011, 133, 10430.
[46] Read de Alaniz, J.; Frazier, C. P.; Engelking, J. R. Org. Lett. 2012, 14, 3620.
[47] Lu, C-D.; Tusun, X. Synlett 2012, 1801.
[48] (a) Johannsen, M.; Jùrgensen, K. A. J. Org. Chem. 1994, 59, 214. (b) Johannsen, M.; Jùrgensen, K. A. J. Org. Chem. 1995, 60, 5979. (c) Srivastava, R. S.; Nicholas, K. M. J. Am. Chem. Soc. 1996, 118, 3311.
(d) Srivastava, R. S.; Nicholas, K. M. J. Am. Chem. Soc. 1997, 119, 3302.
(e) Srivastava, R. S.; Nicholas, K. M. J. Org. Chem. 1994, 59, 5365.
(f) Mùller, E. R.; Jùrgensen, K. A. J. Am. Chem. Soc. 1993, 115, 11814.
(g) Ho, C.-M.; Lau, T.-C. Chem. Commun. 2000, 24, 859.
(h) Hogan, G. A.; Gallo, A. A.; Nicholas, K. M.; Srivastava, R. S. Tetrahedron Lett. 2002, 43, 9505.
(i) Srivastava, R. S. Tetrahedron Lett. 2003, 44, 3271.
[49] Sharpless, K. B. J. Am. Chem. Soc. 1978, 100, 7061.
[50] (a) Cenini, S. Ragaini, F.; Tollari, S.; Paone D. J. Am. Chem. Soc. 1996, 118, 11964.
(b) Srivastava, R. S.; Kolel-Veetil, M. K.; Nicholas, K. M. Tetrahedron Lett. 2002, 43, 931.
[51] Merisor, E.; Conrad, J.; Klaiber, I.; Mika, S.; Beifuss, U. Angew. Chem., Int. Ed. 2007, 46, 3353.
[52] Merisor, E.; Conrad, J.; Klaiber, I.; Mika, S.; Beifuss, U. Synlett 2010, 1766.
[53] Roberts, J. S.; Motherwell, W. B. J. Chem. Soc., Chem. Commun. 1972, 328.
[54] Solomon, D. H.; Moad, G.; Rizzardo, E. Tetrahedron Lett. 1981, 22, 1165.
[55] Haszeldine, R. N.; Barlow, M. G.; Murria, K. W. J. Chem. Soc., Perkin Trans. 1 1980, 1960.
[56] Schenk, C.; De Boer, T. J. Tetrahedron 1979, 35, 147.
[57] Miller, M. J.; Yang, B. Tetrahedron Lett. 2010, 51, 328.
[58] Keck, G. E.; Webb, R. R. J. Am. Chem. Soc. 1981, 103, 3174.
[59] (a) Goodwin, S.; Shoolery, J. N.; Horning, E. C. J. Am. Chem. Soc. 1959, 81, 3736.
(b) Okamoto, T.; Torii, A.; Isogai, Y. Chem. Pharm. Bull. 1968, 16, 1860.
(c) Wildman, W. C.; Bailey, D. T. J. Org. Chem. 1968, 33, 3749.
(d) Wildman, W. C.; Bailey, D. T. J. Am. Chem. Soc. 1970, 92, 5538.
[60] Martin, S. F.; Puckette, T. A.; Colapret, J. A. J. Org. Chem. 1979, 44, 3391.
[61] Wijnberg, J. B. P. A.; Speckamp, W. N. Tetrahedron 1978, 34, 2579.
[62] (a) Matsumura, Y.; Aoyagi, S.; Kibayashi, C. Org. Lett. 2003, 5, 3249.
(b) Matsumura, Y.; Aoyagi, S.; Kibayashi, C. Org. Lett. 2004, 6, 965.
[63] Clive, D. L. J.; Yu, M.; Wang, J.; Yeh, V. S. C.; Kang, S. Chem. Rev. 2005, 105, 4483
[64] Vasella, A.; Zhang, F. Helv. Chim. Acta 2007, 90, 2315.
[65] Vasella, A.; Zhang, F. Helv. Chim. Acta 2008, 91, 2351.
[66] Fokin, A. A.; Yurchenko, A. G.; Rodionov, V. N.; Gunchenko, P. A.; Yurchenko, R. I.; Reichenberg, A.; Wiesner, J.; Hintz, M.; Jomaa, H.; Schreiner, P. R. Org. Lett. 2007, 9, 4379.
[67] Haley, M. M.; Jeffrey, J. L.; McClintock, S. P. J. Org. Chem. 2008, 73, 3288.
[68] Murru, S.; Gallo, A. A.; Srivastava, R. S. J. Org. Chem. 2012, 77, 7119.
[69] Hamer, J.; Macaluso, A. Tetrahedron Lett. 1963, 4, 381.
[70] Seymour, C. A.; Greene, F. D. J. Org. Chem. 1982, 47, 5226.
[71] Davies, A. G.; Schiesser, C. H. Tetrahedron 1991, 47, 1707.
[72] Adam, W.; Krebs, O. J. Org. Chem. 2003, 68, 2420.
[73] (a) Leach, A. G.; Houk, K. N. Chem. Commun. 2002, 12, 1243.
(b) Leach, A. G.; Houk, K. N. J. Am. Chem. Soc. 2002, 124, 14820.
[74] Leach, A. G.; Houk, K. N. J. Am Chem. Soc. 2003, 125, 13825.
[75] Lu, X. Org. Lett. 2004, 6, 2813.
[76] Adam, W.; Wirth, T. Acc. Chem. Res. 1999, 32, 703.
[77] Adam, W.; Bottke, N.; Krebs, O.; Engels, B. J. Am. Chem. Soc. 2001, 123, 5542.
[78] Adam, W.; Bottke, N.; Krebs, O. J. Am. Chem. Soc. 2000, 122, 6791.
[79] Kreae, G.; Felber, H.; Ritter, A.; Ascherl, B.; Vasella, A. Recl. Trav. Chim. Pays-Bas 1986, 105, 295. Adam, W.; Krebs, O. J. Am. Chem. Soc. 2002, 124, 12938.
/
| 〈 |
|
〉 |