

甲亚胺叶立德的1,3-偶极环加成反应合成螺吲哚里西啶类化合物
收稿日期: 2012-07-19
修回日期: 2012-08-08
网络出版日期: 2012-08-21
基金资助
国家自然科学基金(Nos. 20971041, 21172066)资助项目.
Synthesis of Novel Spiro Indolizidine Derivatives via 1,3-Dipolar Cycloaddition of Azomethine Ylide
Received date: 2012-07-19
Revised date: 2012-08-08
Online published: 2012-08-21
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 20971041, 21172066).
李筱芳 , 易荣琼 , 刘彬 , 李志奎 , 于贤勇 , 易平贵 . 甲亚胺叶立德的1,3-偶极环加成反应合成螺吲哚里西啶类化合物[J]. 有机化学, 2012 , 32(12) : 2309 -2314 . DOI: 10.6023/cjoc201207027
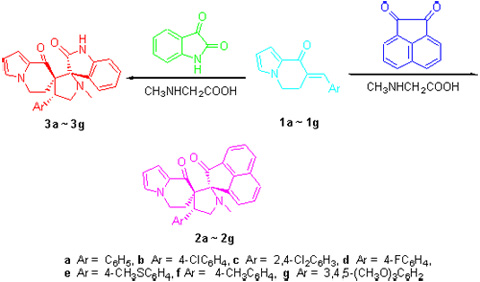
The 1,3-dipolar cycloaddition of azomethine ylide generated in situ from acenaphthenequinone (isatin) and sarcosine to 7-arylmethylidene-6,7-dihydroindolizin-8(5H)-ones afforded novel 4'-aryl-1'-methyl-5'',6''-dihydro-2H,8''H- dispiro[acenaphthylene-1,2'-pyrrolidine-3',7''-indolizine]-2,8''-diones and 4'-aryl-1'-methyl-5'',6''-dihydro-8''H-dispiro[indole-3,2'-pyrrolidine-3',7''-indolizine]-2,8''(1H)-diones in moderate yields. The structures of the products were characterized thoroughly by NMR, IR, MS, elemental analysis together with X-ray crystallographic analysis.
[1] Sridhar, G.; Gunasundari, T.; Raghunathan, R. Tetrahedron Lett. 2007, 48, 319.
[2] Periyasami, G.; Raghunathan, R.; Surendiran, G.; Mathivanan, N. Eur. J. Med. Chem. 2009, 44, 959.
[3] Babu, A. R. S.; Raghunathan, R.; Gayatri, G.; Sastry, G. N. J. Heterocycl. Chem. 2006, 43, 1467.
[4] Sridhar, G.; Raghunathan, R. Synth. Commun. 2006, 36, 21.
[5] Jayashankaran, J.; Manian, R. D. R. S.; Venkatesan, R.; Raghunathan, R. Tetrahedron 2005, 61, 5595.
[6] Kumar, R. S.; Rajesh, S. M.; Perumal, S.; Banerjee, D.; Yogeeswari, P.; Sriram, D. Eur. J. Med. Chem. 2010, 45, 411.
[7] Karthikeyan, S. V.; Bala, B. D.; Raja, V. P. A.; Perumal, S.; Yogeeswari, P.; Sriram, D. Bioorg. Med. Chem. Lett. 2010, 20, 350.
[8] Kumar, R. R.; Loganayaki, B.; Perumal, S. Synth. Commun. 2009, 39, 3197.
[9] Kumar, R. R.; Perumal, S.; Senthilkumar, P.; Yogeeswari, P.; Sriram, D. Eur. J. Med. Chem. 2009, 44, 3821.
[10] Kumar, R. R.; Perumal, S.; Senthilkumar, P.; Yogeeswari, P.; Sriram, D. Tetrahedron 2008, 64, 2962.
[11] Kumar, R. R.; Perumal, S. Tetrahedron 2007, 63, 12220.
[12] Kumar, R. R.; Perumal, S.; Senthilkumar, P.; Yogeeswari, P.; Sriram, D. J. Med. Chem. 2008, 51, 5731.
[13] Cravotto, G.; Giovenzana, G. B.; Pilati, T.; Sisti, M.; Palmisano, G. J. Org. Chem. 2001, 66, 8447.
[14] Michael, J. P. Nat. Prod. Rep. 2005, 22, 603.
[15] Linde, H. H. A. Helv. Chim. Acta 1965, 48, 1822.
[16] Schroder, F.; Franke, S.; Francke, W.; Baumann, H.; Kaib, M.; M. Pasteels, J.; Daloze, D. Tetrahedron 1996, 52, 13539.
[17] Baudoin, O.; Cesario, M.; Guénard D.; Guéritte, F. J. Org. Chem. 2002, 67, 1199.
[18] Jirkovsky, I.; Santroch, G.; Baudy, R.; Oshirot, G. J. Med. Chem. 1987, 30, 388.
[19] Braunholtz, J. T.; Mallion, K. B.; Mann, F. G. J. Chem. Soc. 1962, 4346.
[20] CCDC 832387 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/ data_request/cif.
/
| 〈 |
|
〉 |