

2,3-二氯-5,6-二氰基苯醌/H+作用下4-苯亚甲基-3-异色酮的分子内氧化偶联反应
收稿日期: 2012-07-20
修回日期: 2012-08-28
网络出版日期: 2012-09-03
基金资助
四川省教育厅(No. 12ZA141)及先进功能材料四川省高校重点实验室(No. KFKT2013-01)资助项目.
Intramolecular Oxidative Coupling Reaction of 4-Phenylmethyl-ene-3-isochromanones with 2,3-Dichloro-5,6-dicyanobenzo- quinone as an Oxidant
Received date: 2012-07-20
Revised date: 2012-08-28
Online published: 2012-09-03
Supported by
Project supported by the Department of Education of Sichuan Province (No. 12ZA141) and the Key Laboratory of Advanced Functional Materials of Sichuan Province Higher Education System (No. KFKT2013-01).
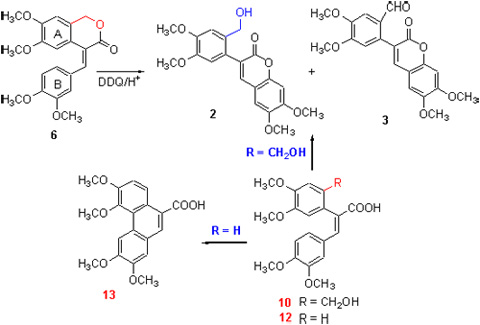
多甲氧基菲-9-甲酸及酯是合成娃儿藤生物碱及其类似物的关键中间体. 以4-(3,4-二甲氧基苯亚甲基)-6,7-二甲氧基-3-异色酮(6)为底物, 2,3-二氯-5,6-二氰基苯醌(DDQ)/CH3SO3H作为氧化体系, 没有得到预期的多甲氧基菲-9-甲酸内酯(1), 意外产物经核磁共振等确定为两个新的3-取代苯基香豆素2, 3. 进一步的实验研究显示: 底物发生分子内脱氢偶联为香豆素2而非菲环化合物1, 是因为异色酮6苯环A上2位取代基的存在所致, 其内酯环开环化合物10以及2,3-二苯基丙烯酸(12)的对比实验印证了该取代基对脱氢偶联反应选择性的影响; 异色酮6氧化偶联为香豆素2的反应机理可能为酸解开环以及途经自由基正离子的脱氢偶联, 香豆素3为DDQ氧化2的前体化合物8所得.
戢得蓉 , 粟立丹 , 张成刚 . 2,3-二氯-5,6-二氰基苯醌/H+作用下4-苯亚甲基-3-异色酮的分子内氧化偶联反应[J]. 有机化学, 2012 , 32(12) : 2334 -2338 . DOI: 10.6023/cjoc201207030
Polymethoxy-substituted phenanthrene-9-carboxylic acid or methylate is key intermediate for synthesis of tylophora alkaloids. (E)-4-(3,4-Dimethoxybenzylidene)-6,7-dimethoxy-1H-isochromen-3(4H)-one (6), oxided by 2,3-dichloro- 5,6-dicyanobenzoquinone (DDQ)/CH3SO3H in CH2Cl2, gave no polymethoxy-substituted phenanthrene derivative (1) but unexpected 3-(2-(hydroxymethyl)-4,5-dimethoxyphenyl)-6,7-dimethoxy-2H-chromen-2-one (2) and 4,5-dimethoxy-2-(6,7-dimethoxy-2-oxo-2H-chromen-3-yl)benzaldehyde (3). Further experiments showed that the steric hindrance of a ring of isochromen-3-one (6) resulted in coumarin (2) not phenanthrene derivative (1), supported by comparative experiments of 2,3-diphenylacrylic acid (10, 12). The possible mechanism of 2 via radical cation and 3 through oxidation of precursor of 2 was proposed based on the result of experiments.

[1] Li, Z.; Jin, Z.; Huang, R. Q. Synthesis 2001, 2365.
[2] Zhang, C. G.; Li, J. J.; Chen, W. M. Chin. J. Med. Chem. 2010, 20, 379 (in Chinese).
(张成刚, 李建军, 陈文明, 中国药物化学杂志, 2010, 20, 379.)
[3] (a) Su, B.; Li, L.; Hu, Y.; Liu, Y.; Wang, Q. M. Adv. Synth. Catal. 2012, 354, 383.
(b) Wang, K. L.; Hu, Y.; Wu, M.; Li, Z.; Liu, Z. H.; Su, B.; Yu, A.; Liu, Y.; Wang, Q. M. Tetrahedron 2010, 66, 9135.
(c) Lu, M. Y.; Wang, K. L.; Cai, F.; Wang, H. Y.; Wang, Q. M. Chin. J. Chem. 2008, 26, 2241.
[4] Finkelstein, J.; Brossi, A. Org. Synth. Coll. 1988, 6, 471.
[5] Tamas, L.; Peter, F.; Földesi, A.; Osz, E.; Prokai, L. Eur. J. Org. Chem. 2002, 17, 2996.
[6] Gu, Y. H.; Xue, K. Tetrahedron Lett. 2010, 51, 192.
[7] Hata, K.; Hamamoto, H.; Shiozaki, Y.; Kita, Y. Chem. Commun. 2005, 19, 2465.
[8] Pandeyx, G.; Krishna, A.; Rao, J. M. Tetrahedron Lett. 1986, 27, 4075.
[9] Lee-ruff, E.; Ablenas, F. J. Can. J. Chem. 1989, 67, 699.
/
| 〈 |
|
〉 |