

流动化学在药物合成中的最新进展
Updated Applications of Flow Chemistry in Pharmaceutical Synthesis
Received date: 2012-08-03
Revised date: 2012-09-26
Online published: 2012-10-08
赵东波 . 流动化学在药物合成中的最新进展[J]. 有机化学, 2013 , 33(02) : 389 -405 . DOI: 10.6023/cjoc201208002
As a further-developing process intensification technology, flow chemistry has achieved a striking development either in academic or industrial area in the past decade. Updated applications of flow chemistry in pharmaceutical synthesis are overviewed. Compared with its early research and development (R&D), current flow chemistry has already shown a lot of breakthroughs. For instance, no more limitations to the proof of feasibility concept for some certain reaction, public development of this technology by more and more distinguished international pharmaceutical companies and quite a few corresponding reports of successful examples about multistep total synthesis of active pharmaceutical ingredients (APIs). Different process categorizations are emphasized with many API syntheses to demonstrate the up-to-date progress of this topic, after a brief introduction of the advantages, existing challenges and the corresponding solutions for flow chemistry.
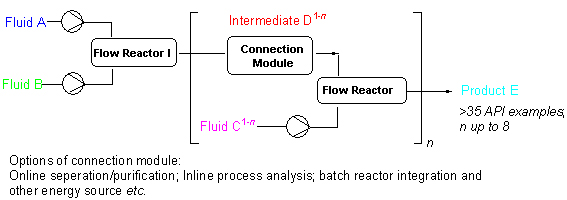
[1] (a) Manz, A.; Graber, N.; Widmer, H. M. Sens. Actuators B 1990, 1, 244.
(b) http://pubs.rsc.org/en/journals/journalissues/lc.
(c) Haswell, S. J.; Middleton, R. J.; O’Sullivan, B.; Skelton, V.; Wattsa, P.; Styringb, P. Chem. Commun. 2001, 391.
(d) http://en.wikipedia.org/wiki/Flow_chemistry.
(e) http://www.jflowchemistry.com.
(f) Elizabeth, F. New Synthetic Technologies in Medicinal Chemistry, RSC publishing, 2012.
[2] Some selected books on Microreaction technology, see: (a) Ehrfeld W.; Hessel V.; Loewe, H. Micro reactors: New Technology for Modern Chemistry, Wiley-VCH, Weinheim, 2000.
(b) Wirth, T. Microreactors in Organic Synthesis and Catalysis, Wiley-VCH, Weinheim, 2008.
(c) Yoshida, J.-I. Flash Chemistry: Fast Organic Synthesis in Microsystems, Wiley-VCH, Weinheim, 2008.
(d) Wiles, C.; Watts, P. Micro Reaction Technology in Organic Synthesis, CRC Press Inc., Boca Raton, 2011.
[3] Some selected international conferences on Microreaction technology, see: (a) International Conference on Microreaction Technology, 1997~2012.
(b) International Symposium on Micro Chemical Process and Synthesis, 2008.
(c) The 2nd Asia-Pacific Chemical and Biological Microfluidics Conference, 2011.
(d) The 3rd European Conference on Microfluidics, 2012.
[4] Some review articles of flow chemistry on pharmaceutical synthesis, see: (a) Kockmann, N.; Roberge, D. M. Chem. Eng. Technol. 2009, 32, 1682.
(b) Malet-Sanz, L.; Susanne, F. J. Med. Chem. 2012, 55, 4062.
(c) Anderson, N. G. Org. Process Res. Dev. 2012, 16, 852.
[5] (a) Schwesinger, N.; Marufke, O.; Qiao, F.; Devant, R.; Wurziger, H. In Process Miniaturization: 2nd International Conference on Microreaction Technology, Eds: Ehrfeld, W.; Rinard, I. H.; Wegeng, R. S., AIChE, New Orleans, 1998, p. 124.
(b) Krummradt, H.; Kopp, U.; Stoldt, J. In Microreaction Technology: 3 rd International Conference on Microreaction Technology, Ed.: Ehrfeld, W., Springer-Verlag, Berlin, 2000, p. 181.
[6] (a) Wörz, O.; Jäckel, K.-P.; Richter, T.; Wolf, A. Chem. Ing. Tech. 2000, 72, 460.
(b) Wörz, O.; Jäckel, K.-P.; Richter, T.; Wolf, A. Chem. Eng. Technol. 2001, 24, 138.
[7] (a) Skelton, V.; Grenway, G. M.; Haswell, S. J.; Styring, P.; Morgan, D. O.; Warrington, B. H.; Wong, S. Y. F. Analyst 2001, 126, 11.
(b) Skelton, V.; Greenway, G. M.; Haswell, S. J.; Styring, P.; Morgan, D. O.; Warrington, B. H.; Wong, S. 4th International Conference on Microreaction Technology, Atlanta, USA, 2001, p. 78.
[8] (a) Wiles, C.; Watts, P.; Haswell, S. J.; Pombo-Villar, E. Lab Chip 2001, 1,100.
(b) Watts, P.; Wiles, C.; Haswell, S. J.; Pombo-Villar, E. Lab Chip 2002, 2,141.
(c) Wiles, C.; Watts, P.; Haswell, S. J.; Pombo-Villar, E. Lab Chip 2004, 4, 171.
[9] (a) Fernandez-Suarez, M.; Wong, S.Y. F.; Warrington, B. H. Lab on Chip 2002, 2,170.
(b) Garcia-Egido, E.; Spikmans, V.; Wong, S. Y. F.; Warrington, B. H. Lab Chip 2003, 3, 67.
[10] (a) Yoshida, J.-I.; Okamoto, H. In Advanced Micro and Nanosystems Vol. 5. Micro Process Engineering, Ed.: Kockmann, N., WILEY-VCH, Weinheim, 2006.
(b) Kawaguchi, T.; Miyata, H.; Ataka, K.; Mae, K.; Yoshida, J.-I. Angew. Chem., Int. Ed. 2005, 44, 2413.
[11] (a) Bohn, L.; Braune, S.; Kotthaus, M.; Kraut, M.; Pöchlauer, P. Vorbach, M.; Wenka, A.; Schubert, K. 9th International Conference on Microreaction Technology, Potsdam, 2006.
(b) Ondrey, G. Chem. Eng. 2011, 118, 16.
[12] (a) Roberge, D. M.; Bieler, N.; Thalmann, M. PharmaChem 2006, 28, 14.
(b) Roberge, D. M. AIChE Spring National Meeting, Houston, TX, 2007, published on CD.
(c) Kockmann, N.; Gottsponer, M.; Zimmermann, B.; Roberge, D. M. Chem. Eur. J. 2008, 14, 7470.
[13] (a) Malet-Sanz, L.; Madrzak, J.; Holvey, R. S.; Underwood, T. Tetrahedron Lett.2009, 50, 7263.
(b) Malet-Sanz, L.; Madrzak, J.; Ley, S. V.; Baxendale, I. R. Org. Biomol. Chem. 2010, 8, 5324.
(c) Grafton, M.; Mansfield, A. C.; Fray, M. J. Tetrahedron Lett. 2010, 51, 1026.
[14] (a) Gutmann, B.; Roduit, J. P.; Roberge, D.; Kappe, C. O. Angew. Chem., Int. Ed. 2010, 49, 7101.
(b) Ye, X.; Johnson, M. D.; Diao, T.; Yates, M. H.; Stahl, S. S. Green Chem. 2010, 12, 1180.
[15] Pelleter, J.; Renaud, F. Org. Process Res. Dev. 2009, 13, 698.
[16] Abele, S.; Ho?ck, S.; Schmidt, G.; Funel, J.-A.; Marti, R. Org. Process Res. Dev. 2012, 16, 1114.
[17] Some selected recent review articles on flow chemistry, see: (a) Wiles, C.; Watts, P. Chem. Commun. 2011, 47, 6512.
(b) Calabrese, G. S.; Pissavini, S. AIChE 2011, 57, 828.
(c) Hartman, R. L.; McMullen, J. P.; Jensen, K. F. Angew. Chem., Int. Ed. 2011, 50, 7502.
(d) Wegner, J.; Ceylan, S.; Kirschning, A. Adv. Synth. Catal. 2012, 354, 17.
[18] Roberge, D. M.; Ducry, L.; Bieler, N.; Cretton, P.; Zimmermann, B. Chem. Eng. Technol. 2005, 28, 318.
[19] Some articles about continuous vs. batch, see: (a) Valera, F. E.; Quaranta, M.; Moran, A.; Blacker, J.; Armstrong, A.; Cabral, J. T.; Blackmond, D. G. Angew. Chem., Int. Ed. 2010, 49, 2478.
(b) Schaber, S. D.; Gerogiorgis, D. I.; Ramachandran, R.; Evans, J. M. B.; Barton, P. I.; Trout, B. L. Ind. Eng. Chem. Res. 2011, 50, 10083. Solid-involved flow process, see:
(c) Kelly, C. B.; Lee, C. X.; Leadbeater, N. E. Tetrahedron Lett. 2011, 52, 263.
(d) Sedelmeier, J.; Ley, S. V.; Baxendale, I. R.; Baumann, M. Org. Lett. 2010, 12, 3618.
(e) Zhao, C.; He, L.; Qiao, S. Z.; Middelberg, A. P. J. Chem. Eng. Sci. 2011, 66, 1463.
(f) Hartman, R. L.; Naber, J. R.; Zaborenko, N.; Buchwald, S. L.; Jensen, K. F. Org. Process Res. Dev. 2010, 14, 1347.
[20] Lee, C.-C.; Sui, G.; Elizarov, A.; Shu, C. J.; Shin, Y.-S.; Dooley, A. N.; Huang, J.; Daridon, A.; Wyatt, P.; Stout, D.; Kolb, H. C.; Witte, O. N.; Satyamurthy, N.; Heath, J. R.; Phelps, M. E.; Quake, S. R.; Tseng, H.-R. Science 2005, 310, 1793.
[21] Baxendale, I. R.; Griffiths-Jones, C. M.; Ley, S. V.; Tranmer, G. F. Synlett 2006, 427.
[22] Zhang, X.; Stefanick, S.; Villani, F. J. Org. Process Res. Dev. 2004, 8, 455.
[23] (a) Kim, H.; Nagaki, A.; Yoshida, J.-i. Nat. Commun. 2011, 2, 264. (b) Gustafsson, T.; So?rensen, H.; Ponte?n, F. Org. Process Res. Dev. 2012, dx.doi.org/10.1021/op200340c.
[24] (a) Hogan, J. Nature 2006, 442, 351.
(b) Hübner, S.; Bentrup, U.; Budde, U.; Lovis, K.; Dietrich, T.; Freitag, A.; Küpper, L.; Jähnisch, K. Org. Process Res. Dev. 2009, 13, 952.
[25] (a) Jähnisch, K.; Dingerdissen, U. Chem. Eng. Technol. 2005, 28, 426.
(b) Lévesque, F.; Seeberger, P. H. Org. Lett. 2011, 13, 5008-5011.
[26] (a) Oelgemöller, M. Chem. Eng. Technol. 2012, 35, 1.
(b) Shinichiro, F.; Nobutake, T.; Yoshida, F.; Yoshida, H.; Doi, T.; Takahashi, T. Chem. Commun. 2010, 46, 8722.
[27] (a) http://en.wikipedia.org/wiki/Lasker_Award.
(b) Dhainaut, J.; Dlubala, A.; Guevel, R.; Medard, A.; Oddon, G.; Raymond, N.; Turconi, J. WO 2011/026865, 2011[Chem. Abstr. 2011, 154, 310801].
(c) Le?vesque, F.; Seeberger, P. H. Angew. Chem., Int. Ed. 2012, 1706.
[28] (a) Kobayashi, J.; Mori, Y.; Okamoto, K.; Akiyama, R.; Ueno, M.; Kitamori, T.; Kobayashi, S. Science 2004, 304, 1305
(b) Jones, R. V.; Godorhazy, L.; Varga, N.; Szalay, D.; Urge, L.; Darvas, F. J. Comb. Chem. 2006, 8, 110.
(c) http://www.thalesnano.com/products/h-cube
[29] (a) Ley, S. V.; Schucht, O.; Thomas, A. W.; Murray, P. J. J. Chem. Soc., Perkin Trans. 1 1999, 1251.
(b) Baxendale, I. R.; Deeley, J.; Griffiths-Jones, C. M.; Ley, S. V.; Saaby, S.; Tranmer, G. K. Chem. Commun. 2006, 2566.
[30] (a) Capdeville, R.; Buchdunger, E.; Zimmermann, J.; Matter, A. Nat. Rev. Drug Discovery 2002, 1, 493.
(b) Arora, A.; Scholar, E. M. J. Pharmacol. Exp. Ther. 2005, 315, 971.
(c) Hopkin, M. D.; Baxendale, I. R.; Ley, S. V. Chem. Commun. 2010, 46, 2450.
[31] (a) Willging, E. M. Anal. Chem. 1987, 59, 938.
(b) Loupy, A. Microwaves in Organic Synthesis, John Wiley, New York, 2002, p. 61.
[32] (a) Torborg, C.; Beller, M. Adv. Synth. Catal. 2009, 351, 3027.
(b) Glasnova, T. N.; Kappe, C. O. Adv. Synth. Catal. 2010, 352, 3089.
(c) Gustafsson, T.; Pontn, F.; Seeberger, P. H. Chem. Commun. 2008, 1100.
(d) Bedore, M. W.; Zaborenko, N.; Jensen, K. F.; Jamison, T. F. Org. Process Res. Dev. 2010, 14, 432.
(e) Glasnov, T. N.; Findenig, S.; Kappe, C. O. Chem. Eur. J. 2009, 15, 1001.
(f) Gutmann, B.; Roduit, J.-P.; Roberge, D.; Kappe, C. O. Angew. Chem., Int. Ed. 2010, 49, 7101.
[33] (a) Kim, E. G.; Schmidt, K.; Caseri, W. R.; Kreouzis, T.; Stingelin-Stutzmann, N.; Bredas, J. L. Adv. Mater. 2006, 18, 2039.
(b) Caseri, W. R.; Platinum Met. Rev. 2004, 48, 91.
(c) Pedrick, E. A.; Leadbeater, N. E. Inorg. Chem. Commun. 2011, 14, 481.
[34] Bogdan, A. R.; Poe, S. L.; Kubis, D. C.; Broadwater, S. J.; McQuade, D. T. Angew. Chem., Int. Ed. 2009, 48, 8547.
[35] Baumann, M.; Baxendale, I. R.; Brasholz, M.; Hayward, J. J.; Ley, S. V.; Nikbin, N. Synlett 2011, 1375.
[36] Cervera-Padrell, A. E.; Nielsen, J. P.; Pedersen, M. J.; Christensen, K. M.; Mortensen, A. R.; Skovby, T.; Dam-Johansen, K.; Kiil, S.; Gernaey, K. V. Org. Process Res. Dev. 2012, 16, 901.
[37] (a) Moorhouse, A. D.; Moses, J. E. Chem. Soc. Rev. 2007, 36, 1249.
(b) Baxendale, I. R.; Ley, S. V.; Mansfield, A. C.; Smith, C. D. Angew. Chem., Int. Ed. 2009, 48, 4017.
[38] (a) Daugan, A.; Grondin, P.; Ruault, C.; Le Monnier de Gouville, A.-C.; Coste, H.; Linget, J.-M.; Kirilovsky, J.; Hyafil, F.; Labaudinière, R. J. Med. Chem. 2003, 46, 4533.
(b) Mezencev, R.; Updegrove, T.; Kutschy, P.; Repovská, M.; McDonald, J. F. J. Nat. Med. 2011, 65, 488.
(c) Moody, C. J.; Roffey, J. R. A.; Swann, E.; Lockyer, S.; Houlbrook, S.; Stratford, I. J. Anti-Cancer Drugs 1999, 10, 577.
(d) Pagano, N; Heil, M. L.; Cosford, N. D. P. Synthesis 2012, 2537.
[39] Bonrath, W.; Karge, R.; Netscher, T. J. Mol. Catal. B: Enzym. 2002, 19~20, 67.
[40] Schwalbe, T.; Autze, V.; Wille, G. Chimia 2002, 56, 636.
[41] Choe, J.; Song, K.-H.; Kwon, Y. 4th Asia-Pacific Chemical Reaction Engineering Symposium, Gyeongju, Korea, 2005, p. 435.
[42] Sugimoto, A.; Sumino, Y.; Takagi, M.; Fukuyama, T.; Ryu, I. Tetrahedron Lett. 2006, 47, 6197.
[43] Pitts, M. R.; McCormack, P.; Whittall, J. Tetrahedron 2006, 62, 4705.
[44] Gustafsson, T.; Pontén, F.; Seeberger, P. H. Chem. Commun. 2008, 1100.
[45] LaPorte, T. L.; Hamedi, M.; DePue, J. S.; Shen, L.; Watson, D.; Hsieh, D. Org. Process Res. Dev. 2008, 12, 956.
[46] Grant, D.; Dahl, R.; Cosford, N. D. P. J. Org. Chem. 2008, 73, 7219.
[47] Kopach, M. E.; Murray, M. M.; Braden, T. M.; Kobierski, M. E.; Williams, O. L. Org. Process Res. Dev.2009, 13, 152.
[48] Buddoo, S.; Siyakatshana, N.; Zeelie, B.; Dudas, J. Chem. Eng. Process. 2009, 48, 1419.
[49] Tanaka, K.; Miyagawa, T.; Fukase, K. Synlett 2009, 1571.
[50] Tanaka, K.; Fukase, K. Org. Process Res. Dev.2009, 13, 983.
[51] Bogdan, A. R.; James, K. Chem. Eur. J. 2010, 16, 14506.
[52] Brasholz, M.; Johnson, B. A.; Macdonald, J. M.; Polyzos, A.; Tsanaktsidis, J.; Saubern, S.; Holmes, A. B.; Ryan, J. H. Tetrahedron 2010, 66, 6445.
[53] Qian, Z.; Baxendale, I. R.; Ley, S. V. Synlett 2010, 505.
[54] Brasholz, M.; Macdonald, J. M.; Saubern, S.; Ryan, J. H.; Holmes, A. B. Chem. Eur. J. 2010, 16, 11471.
[55] Qian, Z.; Baxendale, I. R.; Ley, S. V. Chem. Eur. J. 2010, 16, 12342.
[56] Noel, T.; Kuhn, S; Musacchio, A. J.; Jensen, K. F.; Buchwald, S. L. Angew. Chem., Int. Ed. 2011, 50, 5943.
/
| 〈 |
|
〉 |