

过渡金属催化醇与胺有氧脱水反应及相关研究进展
收稿日期: 2012-08-17
修回日期: 2012-09-22
网络出版日期: 2012-10-08
基金资助
温州大学引进人才科研启动经费、国家自然科学基金(No. 20902070);教育部第40批留学回国人员科研启动金、浙江省自然科学基金(No. Y4100579)和钱江人才计划D类(No. QJD0902004)资助项目.
Recent Advances of Transition Metal-Catalyzed Aerobic Dehydrative Reactions of Alcohols and Amines and Related Researches
Received date: 2012-08-17
Revised date: 2012-09-22
Online published: 2012-10-08
Supported by
Project supported by the Startup Funding of Wenzhou University, the National Natural Science Foundation of China (No. 20902070), the Scientific Research Foundation for the Returned Overseas Chinese Scholars of State Education Ministry, the Natural Science Foundation of Zhejiang Province (No. Y4100579) and the Qianjiang Talents Program of Zhejiang Province (No. QJD0902004).
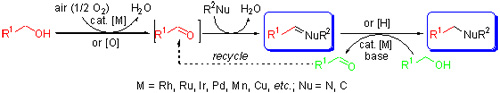
与其他胺和酰胺衍生物的合成方法相比, 过渡金属催化醇与各类胺和酰胺的脱水N-烷基化反应是一种相对绿色、原子经济性较高的方法, 一般被称为“借氢”或“氢自动转移”反应及其方法学. 近年来, 在空气氛围下过渡金属催化醇与胺和酰胺的有氧脱水N-烷基化反应, 可使用更稳定的金属催化剂、可在无配体、空气等更温和简单的条件下进行, 也引起了人们的极大关注. 主要介绍近年来过渡金属催化下醇与胺和酰胺在空气或者氧化剂作用下构建C—N, C=N键合成胺和酰胺衍生物以及亚胺类化合物的有氧脱水反应进展情况, 同时也对相关有氧脱水C-烷基化反应进行简单介绍. 相关反应的机理研究也将作适当讨论.
徐清 , 李强 . 过渡金属催化醇与胺有氧脱水反应及相关研究进展[J]. 有机化学, 2013 , 33(01) : 18 -35 . DOI: 10.6023/cjoc201208016
In comparison with other methods, transition metal-catalyzed dehydrative N-alkylation of amines and amides with alcohols, commonly known as the borrowing hydrogen or hydrogen autotransfer reactions and the methodology, is a comparatively green and atom-economic method for the synthesis of the useful amine and amide derivatives. Recently, transition metal-catalyzed aerobic dehydrative N-alkylation method has also attracted much attention, for the reactions can be readily conducted under milder and simpler conditions by using the more stable metal catalysts under the air atmosphere. In this review, we summarize the recent advances of transition metal-catalyzed aerobic dehydrative C—N and C=N bond forming reactions of alcohols with amines and amides for the synthesis of the amine and amide derivatives and imines, as well as those of the related aerobic dehydrative C-alkylation reactions. Mechanisms of the above reactions are also discussed.

[1] (a) Brooks, G. T.; Roberts, T. R. Pesticide Chemistry and Bioscience, Royal Society of Chemistry, Cambridge, U.K., 1999.
(b) McGuire, J. L. Pharmaceuticals: Classes, Therapeutic Agents, Areas of Application, Vols. 1~4, Wiley-VCH, Weinheim, Germany, 2000.
(c) Hili, R.; Yudin, A. K. Nat. Chem. Biol. 2006, 2, 284.
(d) Cox, E. D.; Cook, J. M. Chem. Re v. 1995, 95, 1797.
(e) Humphrey, J. M.; Chamberlin, A. R. Chem. Rev. 1997, 97, 2243.
(f) Hansch, C.; Sammes, P. G.; Taylor, J. B. Comprehensive Medicinal Chemistry, Vol. 2, Pergamon Press, Oxford, 1990, Chapter 7.1.
(g) Connor, E. E. Prim. Care Update Ob/Gyns 1998, 5, 32.
(h) Kleemann, A.; Engel, J.; Kutscher, B.; Reichert, D. Pharmaceutical Substances, Synthesis, Patents, Applications, Thieme, Stuggart, 1999.
(i) Park, S.-Y.; Fung, P.; Nishimura, N.; Jensen, D. R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T. F.; Alfred, S. E.; Bonetta, D.; Finkelstein, R.; Provart, N. J.; Desveaux, D.; Rodriguez, P. L.; McCourt, P.; Zhu, J.-K.; Schroeder, J. I.; Volkman, B. F.; Cutler, S. R. Science 2009, 324, 1068.
[2] (a) Bloch, R. Chem. Re v. 1998, 98, 1407.
(b) Yet, L. Angew. Chem., Int. Ed. 2001, 40, 875.
(c) Ellman, J. A.; Owens, T. D.; Tang, T. P. Acc. Chem. Res. 2002, 35, 984.
(d) Taggi, A. E.; Hafez, A. M.; Lectka, T. Acc. Chem. Res. 2003, 36, 10.
(e) Córdova, A. Acc. Chem. Res. 2004, 37, 102.
(f) Ma, J.-A. Chem. Soc. Rev. 2006, 35, 630.
(g) Dömling, A. Chem. Rev. 2006, 106, 17.
(h) Erkkilä, A.; Majander, I.; Pihko, P. M. Chem. Rev. 2007, 107, 5416.
(i) Gawronski, J.; Wascinska, N.; Gajewy, J. Chem. Rev. 2008, 108, 5227.
(j) Yamada, K.-I.; Tomioka, K. Chem. Re v. 2008, 108, 2874.
(k) Merino, P.; Marqués-López, E.; Herrera, R. P. Adv. Synth. Catal. 2008, 350, 1195.
(l) Ordóñez, M.; Rojas-Cabrera, H.; Cativiela, C. Tetrahedron 2009, 65, 17.
(m) Martin, S. F. Pure Appl. Chem. 2009, 81, 195.
(n) de Armas, P.; Tejedor, D.; Garía-Tellado, F. Angew. Chem., Int. Ed. 2010, 49, 1013.
(o) Li, C.-J. Acc. Chem. Res. 2010, 43, 581.
(p) Akhmetova, V. R.; Khabibullina, G. R.; Rakhimova, E. B.; Vagapov, R. A.; Khairullina, R. R.; Niatshina, Z. T.; Murzakova, N. N. Mol. Diversity 2010, 14, 463.
(q) Yin, B.; Zhang, Y.; Xu, L.-W. Synthesis 2010, 3583.
(r) Kobayashi, S.; Mori, Y.; Fossey, J. S.; Salter, M. M. Chem. Rev. 2011, 111, 2626.
(s) Xie, J.-H.; Zhu, S.-F.; Zhou, Q.-L. Chem. Rev. 2011, 111, 1713.
(t) Adrio, J.; Carretero, J. C. Chem. Commun. 2011, 47, 6784.
(u) Marques, C. S.; Burke, A. J. ChemCatChem 2011, 3, 635.
(v) Ramadhar, T. R.; Batey, R. A. Synthesis 2011, 1321.
(w) Nielsen, M.; Worgull, D.; Zweifel, T.; Gschwend, B.; Bertelsen, S.; Jøgensen, K. A. Chem. Commun. 2011, 47, 632.
[3] (a) Hartwig, J. F. Acc. Chem. Res. 2008, 41, 1534.
(b) Surry, D. S.; Buchwald, S. L. Angew. Chem., Int. Ed. 2008, 47, 6338.
(c) Evano, G.; Blanchard, N.; Toumi, M. Chem. Rev. 2008, 108, 3054.
[4] (a) Tripathi, R. P.; Verma, S. S.; Pandey, J.; Tiwari, V. K. Curr. Org. Chem. 2008, 12, 1093.
(b) Burkhardt, E. R.; Karl Matos, K. Chem. Rev. 2006, 106, 2617.
(c) Baxter, E. W.; Reitz, A. B. In Organic Reactions, Vol. 59, Ed.: Overman, L. E., Wiley, Singapore, 2002.
[5] (a) Müller, T. E.; Hultzsch, K. C.; Yus, M.; Foubelo, F.; Tada, M. Chem. Rev. 2008, 108, 3795.
(b) Severin, R.; Doye, S. Chem. Soc. Rev. 2007, 36, 1407.
(c) Müller, T. E.; Beller, M. Chem. Rev. 1998, 98, 675.
[6] (a) Veige, A. S. Polyhedron 2008, 27, 3177.
(b) Beccalli, E. M.; Broggini, G.; Martinelli, M.; Sottocornola, S. Chem. Rev. 2007, 107, 5318.
(c) Evano, G.; Coste, A.; Jouvin, K. Angew. Chem., Int. Ed. 2010, 49, 2840.
(d) DeKorver, K. A.; Li, H.; Lohse, A. G.; Hayashi, R.; Lu, Z.; Zhang, Y.; Hsung, R. P. Chem. Rev. 2010, 110, 5064.
[7] (a) Salvatore, R. N.; Yoon, C. H.; Jung, K. W. Tetrahedron 2001, 57, 7785.
(b) Chiappe, C.; Pieraccini, D. Green Chem. 2003, 5, 193.
[8] Nef, J. U. Liebigs Ann. Chem. 1901, 318, 137.
[9] Guillena, G.; Ramón, D. J.; Yus, M. Chem. Re v. 2010, 110, 1611.
[10] (a) Grigg, R.; Mitchell, T. R. B.; Sutthivaiyakit, S.; Tongpenyai, N. J. Chem. Soc., Chem. Commun. 1981, 611.
(b) Watanabe, Y.; Tsuji, Y.; Ohsugi, Y. Tetrahedron Lett. 1981, 22, 2667.
(c) Maruhashi, S.-I.; Kondo, K.; Hakata, T. Tetrahedron Lett. 1982, 23, 229.
[11] (a) Watson, A. J. A.; Williams, J. M. J. Science 2010, 329, 635.
(b) Nixon, T. D.; Whittlesey, M. K.; Williams, J. M. J. Dalton Trans. 2009, 753.
(c) Hamid, M. H. S. A.; Slatford, P. A.; Williams, J. M. J. Ad v. Synth. Catal. 2007, 349, 1555.
(d) Suzuki, T. Chem. Rev. 2011, 111, 1825.
(e) Bähn, S.; Imm, S.; Neubert, L.; Zhang, M.; Neumann, H.; Beller, M. ChemCatChem 2011, 3, 1853.
(f) Crabtree, R. H. Organometallics 2011, 30, 17.
(g) Dobereiner, G. E.; Crabtree, R. H. Chem. Re v. 2010, 110, 681.
(h) Guillena, G.; Ramón, D. J.; Yus, M. Angew. Chem., Int. Ed. 2007, 46, 2358.
(i) Fujita, K.-I.; Yamaguchi, R. Synlett 2005, 560.
[12] (a) Emer, E.; Sinisi, R.; Capdevila, M. G.; Petruzziello, D.; De Vincentiis, F.; Cozzi, P. G. Eur. J. Org. Chem. 2011, 647 and references cited therein.
(b) Guérinot, A.; Reymond, S.; Cossy, J. Eur. J. Org. Chem. 2011, 647.
(c) Qin, H.; Yamagiwa, N.; Matsunaga, S.; Shibasaki, M. Angew. Chem., Int. Ed. 2007, 46, 409.
(d) Zhan, Z.-P.; Yang, W.-Z.; Yang, R.-F.; Yu, J.-L.; Li, J.-P.; Liu, H.-J. Chem. Commun. 2006, 3352.
(e) Terrasson, V.; Marque, S.; Georgy, M.; Campagne, J.-M.; Prim, D. Adv. Synth. Catal. 2006, 348, 2063.
(f) Zhan, Z.-P.; Yu, J.-L.; Liu, H.-J.; Cui, Y.-Y.; Yang, R.-F.; Yang, W.-Z.; Li, J.-P. J. Org. Chem. 2006, 71, 8298.
(g) Zhao, Y.; Foo, S. W.; Saito, S. Angew. Chem., Int. Ed. 2011, 50, 3006.
(h) Tsai, C.-Y.; Sung, R.; Zhuang, B.-R.; Sung, K. Tetrahedron 2010, 66, 6869.
(i) Sreedhar, B.; Reddy, P. S.; Reddy, M. M.; Neelima B.; Arundhathi, R. Tetrahedron Lett. 2007, 48, 8174.
(j) Das, B.; Reddy, P. R.; Sudhakar, C.; Lingaiah, M. Tetrahedron Lett. 2011, 52, 3521.
(k) Ozawa, F.; Okamoto, H.; Kawagishi, S.; Yamamoto, S.; Minami, T.; Yoshifuji, M. J. Am. Chem. Soc. 2002, 124, 10968.
(l) Ohshima, T.; Miyamoto, Y.; Ipposhi, J.; Nakahara, Y.; Utsunomiya, M.; Mashima, K. J. Am. Chem. Soc. 2009, 131, 14317.
(m) Utsunomiya, M.; Miyamoto, Y.; Ipposhi, J.; Ohshima, T.; Mashima, K. Org. Lett. 2007, 9, 3371.
(n) Tao, Y.; Wang, B.; Wang, B.; Qu, L.; Qu, J. Org. Lett. 2010, 12, 2726.
(o) Roggen, M.; Carreira, E. M. J. Am. Chem. Soc. 2010, 132, 11917.
[13] Blackburn, L.; Taylor, R. J. K. Org. Lett. 2001, 3, 1637.
[14] Kanno, H.; Taylor, R. J. K. Tetrahedron Lett. 2002, 43, 7337.
[15] Guerin, C.; Bellosta, V.; Guillamot, G.; Cossy, J. Org. Lett. 2011, 13, 3534.
[16] Guerin, C.; Bellosta, V.; Guillamot, G.; Cossy, J. Eur. J. Org. Chem. 2012, 15, 2990.
[17] Likhar, P. R.; Arundhathi, R.; Kantam, M. L. Eur. J. Org. Chem. 2009, 5383.
[18] Shi, F.; Tse, M. K.; Cui, X.; Goerdes, D.; Michalik, D.; Thurow, K.; Deng, Y.; Beller, M. Angew. Chem., Int. Ed. 2009, 48, 5912.
[19] (a) Martínez-Asencio, A.; Ramón, D. J.; Yus, M. Tetrahedron Lett. 2010, 51, 325.
(b) Martínez-Asencio, A.; Ramón, D. J.; Yus, M. Tetrahedron 2011, 67, 3140.
[20] Cui, X.; Shi, F.; Tse, M. K.; Goerdes, D.; Thurow, K.; Beller, M.; Deng, Y. Adv. Synth. Catal. 2009, 351, 2949.
[21] Feng, S. L.; Liu, C. Z.; Li, Q.; Yu, X. C.; Xu, Q. Chin. Chem. Lett. 2011, 22, 1021.
[22] Liu, C.; Liao, S.; Li, Q.; Feng, S.; Sun, Q.; Yu, X.; Xu, Q. J. Org. Chem. 2011, 76, 5759 and references cited therein.
[23] (a) Sheldon, R. A.; Arends, I. W. C. E.; Brink, G.-J. T.; Dijksman, A. Acc. Chem. Res. 2002, 35, 774.
(b) Gligorich, K. M.; Sigman, M. S. Chem. Commun. 2009, 3854.
(c) Stahl, S. S. Angew. Chem., Int. Ed. 2004, 43, 3400.
(d) Naota, T.; Takaya, H.; Murahashi, S.-I. Chem. Rev. 1998, 98, 2599.
(e) Muzart, J. Tetrahedron 2003, 59, 5789.
(f) Arita, S.; Koike, T.; Kayaki, Y.; Ikariya, T. Angew. Chem., Int. Ed. 2008, 47, 2447.
(g) Zhang, J.; Li, S.; Fu, X.; Wayland, B. B. Dalton Trans. 2009, 3661.
(h) Izumi, A.; Obora, Y.; Sakaguchi, S.; Ishii, Y. Tetrahedron Lett. 2006, 47, 9199.
[24] (a) Chohan, Z. H.; Shad, H. A.; Nasim, F.-H. Appl. Organomet. Chem. 2009, 23, 319 and references therein.
(b) Cejudo-Marín, R.; Alzuet, G.; Ferrer, S.; Borrás, J. Inorg. Chem. 2004, 43, 6805.
(c) Zhang, T.; Wang, W.; Gu, X.; Shi, M. Organometallics 2008, 27, 753.
(d) Liang, J.; Lipscomb, W. N. Biochemistry 1989, 28, 9724.
(e) Evelhoch, J. L. E.; Bocian, D. F.; Sudmeier, J. L. Biochemistry 1981, 20, 4951.
(f) Dimroth, J.; Keilitz, J.; Schedler, U.; Schomäcker, R.; Haag, R. Adv. Synth. Catal. 2010, 352, 2497.
[25] (a) Watanabe, Y.; Morisaki, Y.; Kondo, T.; Mitsudo, T. J. Org. Chem. 1996, 61, 4214.
(b) Watanabe, Y.; Tsuji, Y.; Ige, H.; Ohsugi, Y.; Ohta, T. J. Org. Chem. 1984, 49, 3359.
(c) Fujita, K.-I.; Enkoi, Y.; Yamaguchi, R. Tetrahedron 2008, 64, 1943.
[26] Yu, X.; Jiang, L.; Li, Q.; Xie, Y. ; Xu, Q. Chin. J. Chem. 2012, 30, 2322.
[27] (a) Layer, R. W. Chem. Rev. 1963, 63, 489.
(b) Sprung, M. M. Chem. Rev. 1940, 26, 297.
(c) Patai, S. The Chemistry of the Carbon—Nitrogen Double Bond (Chemistry of Functional Goups), Wiley-Interscience, New York, 1970.
(d) Adams, J. P. J. Chem. Soc., Perkin Trans. 1 2000, 125.
[28] (a) Gladiali, S.; Alberico, E. Chem. Soc. Rev. 2006, 35, 226.
(b) Samec, J. S. M.; B?ckvall, J.-E.; Andersson, P. G.; Brandt, P. Chem. Soc. Rev. 2006, 35, 237.
(c) Saluzzo, C.; Lemaire, M. Ad v . Synth. Catal. 2002, 344, 915.
(d) Noyori, R.; Hashiguchi, S. Acc. Chem. Res. 1997, 30, 97.
(e) Krische, M. J.; Sun, Y. Special issue on hydrogenation and transfer hydrogenation. Acc. Chem. Res. 2007, 40, issue 12.
[29] (a) Kwon, M. S.; Kim, S.; Park, S.; Bosco, W.; Chidrala, R. K.; Park, J. J. Org. Chem. 2009, 74, 2877.
(b) Corma, A.; Ródenas, R.; Sabater, M. J. Chem. Eur. J. 2010, 16, 254.
(c) Zhang, Y.; Qi, X.; Cui, X.; Shi, F.; Deng, Y. Tetrahedron Lett. 2011, 52, 1334.
[30] Martínez-Asencio, A.; Yus, M.; Ramón, D. J. Synthesis 2011, 3730.
[31] Kawahara, R.; Fujita, K.-I.; Yamaguchi, R. Adv. Synth. Catal. 2011, 353, 1161.
[32] Ohta, H.; Yuyama, Y.; Uozumi, Y.; Yamada, Y. M. A. Org. Lett. 2011, 13, 3892.
[33] Yu, X.; Liu, C.; Jiang, L.; Xu, Q. Org. Lett. 2011, 13, 6184.
[34] (a) Gonzalez-Arellano, C.; Yoshida, K.; Luque, L.; Gai, P. L. Green Chem. 2010 12, 1281.
(b) Martínez, M.; Ramón, D. J.; Yus, M. Org. Biomol. Chem. 2009, 7, 2176.
(c) Valotl, F.; Fachel, F.; Jacquot, R.; Spagnol, M.; Lemairel, M. Tetrahedron Lett. 1999, 40, 3689.
[35] (a) Shi, F.; Tse, M. K.; Zhou, S.; Pohl, M. M.; Radnik, J.; Hübner, S.; J?hnisch, K.; Brückner, A.; Beller, M. J. Am. Chem. Soc. 2009, 131, 1775.
(b) Cano, R.; Ramón, D. J.; Yus, M. J. Org. Chem. 2011, 76, 5547.
(c) Yamaguchi, K.; He, J.; Oishi, T.; Mizuno, N. Chem. Eur. J. 2010, 16, 7199.
(d) He, J.; Kim, J. W.; Yamaguchi, K.; Mizuno, N. Angew. Chem., Int. Ed. 2009, 48, 9888.
(e) Cui, X.; Zhang, Y.; Shi, F.; Deng, Y. Chem. Eur. J. 2011, 17, 1021.
(f) He, L.; Lou, X.-B.; Ni, J.; Liu, Y.-M.; Cao, Y.; He, H.-Y.; Fan, K.-N. Chem. Eur. J. 2010, 16, 13965.
[36] (a) Li, C.-J.; Li, Z. Pure Appl. Chem. 2006, 78, 935.
(b) Li, C.-J. Acc. Chem. Res. 2009, 42, 335.
(c) Scheuermann, C. J. Chem. Asian J. 2010, 5, 436.
(d) Li, Z.; Bohle, S.; Li, C.-J. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 8928.
[37] (a) Semmelhack, M. F.; Schmid, C. R.; Cortes, D. A.; Chou, C. S. J. Am. Chem. Soc. 1984, 106, 3374.
(b) Markó, I. E.; Giles, P. R.; Tsukazaki, M.; Brown, S. M.; Urch, C. J. Science 1996, 274, 2044.
(c) Markó, I. E.; Gautier, A.; Dumeunier, R.; Doda, K.; Philippart, F.; Brown, S. M.; Urch, C. J. Angew. Chem., Int. Ed. 2004, 43, 1588.
(d) Gamez, P.; Arends, I. W. C. E.; Reedijk, J.; Sheldon, R. A. Chem. Commun. 2003, 2414.
(d) Hoover, J. M.; Stahl, S. S. J. Am. Chem. Soc. 2011, 133, 16901.
[38] (a) Wendlandt, A. E.; Suess, A. M.; Stahl, S. S. Angew. Chem., Int. Ed. 2011, 50, 11062.
(b) Liu, C.; Zhang, H.; Shi, W.; Lei, A. Chem. Rev. 2011, 111, 1780.
(c) Klussmann, M.; Sureshkumar, D. Synthesis 2011, 353.
(d) Zhao, Z.; Peng, F. Angew. Chem., Int. Ed. 2010, 49, 9566.
(e) Stefani, H. A.; Guarezemini, A. S.; Cella, R. Tetrahedron 2010, 66, 7871.
[39] Li, Q.; Fan, S.; Sun, Q.; Tian, H.; Yu, X.; Xu, Q. Org. Biomol. Chem. 2012, 10, 2966 and references cited therein.
[40] (a) Deutsch, C.; Krause, N.; Lipshutz, B. H. Chem. Re v. 2008, 108, 2916.
(b) Rendler, S.; Oestreich, M. Angew. Chem., Int. Ed. 2007, 46, 498.
(c) Lipshutz, B. H. Synlett 2009, 509.
[41] Jiang, L.; Jin, L.; Tian, H.; Yuan, X.; Yu, X.; Xu, Q. Chem. Commun. 2011, 47, 10833.
[42] Tian, H.; Yu, X.; Li, Q.; Wang, J.; Xu, Q. Adv. Synth. Catal. 2012, 354, 2671.
[43] Yamada, Y. M. A.; Uozumi, Y. Org. Lett. 2006, 8, 1375.
[44] Yamada, Y. M. A.; Uozumi, Y. Tetrahedron 2007, 63, 8492.
[45] Allen, L. J.; Crabtree, R. H. Green Chem. 2010, 12, 1362.
[46] (a) Thomé, I.; Nijs, A.; Bolm, C. Chem. Soc. Rev. 2012, 41, 979.
(b) Leadbeater, N. E. Nat. Chem. 2010, 2, 913.
(c) Buchwald, S. L.; Bolm, C. Angew. Chem., Int. Ed. 2009, 48, 5586.
[47] Tang, G.; Cheng, C.-H. Adv. Synth. Catal. 2011, 353, 1918.
[48] Liao, S.; Yu, K.; Li, Q.; Tian, H.; Zhang, Z.; Yu, X.; Xu, Q. Org. Biomol. Chem. 2012, 10, 2973 and references cited therein.
[49] (a) Palacios, F.; Alonso, C.; Aparicio, D.; Rubiales, G.; de los Santos, J. M. Tetrahedron 2007, 63, 523.
(b) Fresneda, P. M.; Molina, P. Synlett 2004, 1.
[50] (a) Largeron, M.; Fleury, M.-B. Angew. Chem., Int. Ed. 2012, 51, 5409.
(b) Lang, X.; Ji, H.; Chen, C.; Ma, W.; Zhao, J. Angew. Chem., Int. Ed. 2011, 50, 3934.
(c) Jiang, G.; Chen, J.; Huang, J.-S.; Che, C.-M. Org. Lett. 2009, 11, 4568.
(d) Samec, J. S. M.; Éll, A. H.; Bäckvall, J.-E. Chem. Eur. J. 2005, 11, 2327.
[51] Schiff, H. Annals 1864, 131, 118.
[52] Medvedeva, A. S.; Mareev, A. V.; Borisova, A. I.; Afonin, A. V. ARKIVOC 2003, 13, 157.
[53] Yusubov, M. S.; Chi, K.-W.; Park, J. Y.; Karimovc, R.; Zhdankinc, V. V. Tetrahedron Lett. 2006, 47, 6305.
[54] (a) Gnanaprakasam, B.; Zhang, J.; Milstein, D. Angew. Chem., Int. Ed. 2010, 49, 1468.
(b) Maggi, A.; Madsen, R. Organometallics 2012, 31, 451.
(c) Esteruelas, M. A.; Honczek, N.; Oliván, M.; Onate, E.; Valencia, M. Organometallics 2011, 30, 2468.
(d) Cano, R.; Ramón, D. J.; Yus, M. J. Org. Chem. 2011, 76, 5547.
(e) Shiraishi, Y.; Ikeda, M.; Tsukamoto, D.; Tanaka, S.; Hiraia, T. Chem. Commun. 2011, 47, 4811.
[55] Sun, H.; Su, F.-Z.; Ni, J.; Cao, Y.; He, H.-Y.; Fan, K.-N. Angew. Chem., Int. Ed. 2009, 48, 4390.
[56] Kim, J. W.; He, J.; Yamaguchi, K.; Mizuno, N. Chem. Lett. 2009, 38, 920.
[57] Kegnaes, S.; Mielby, J.; Mentzel, U. V.; Christensen, C. H.; Riisager, A. Green Chem. 2010, 12, 1437.
[58] He, W.; Wang, L.; Sun, C.; Wu, K.; He, S.; Chen, J.; Wu, P.; Yu, Z. Chem. Eur. J. 2011, 17, 13308.
[59] Kang, Q.; Zhang, Y. Green Chem. 2012, 14, 1016.
[60] Sithambaram, S.; Kumar R.; Son, Y.-C.; Suib, S. L. J. Catal. 2008, 253, 269.
[61] Kwon, M. S.; Kim, N.; Seo, S. H.; Park, I. S.; Cheedrala, R. K.; Park, J. Angew. Chem., Int. Ed. 2005, 44, 6913.
[62] Kim, S.; Bae, S. W.; Lee, J. S.; Park, J. Tetrahedron 2009, 65, 1461.
[63] Xu, Q.; Tian, H.; Jin, L. CN 102775288, 2012 [Chem. Abstr. 2012, 158, 11329].
/
| 〈 |
|
〉 |