

2-苄硫(砜)基-5-甲基-7-取代苄氧基-1,2,4-三唑并[1,5-a]嘧啶类衍生物的合成及抑菌活性研究
收稿日期: 2012-08-29
修回日期: 2012-10-15
网络出版日期: 2012-10-24
基金资助
贵州省优秀科技教育人才省长专项资金(No. 200817)资助项目.
Synthesis and Fungicidal Activities of 2-Benzylthio(sulfonyl)-5- methyl-7-substituted benzyloxy-1,2,4-triazolo[1,5-a] pyrimidine Derivatives
Received date: 2012-08-29
Revised date: 2012-10-15
Online published: 2012-10-24
Supported by
Project supported by the Governor’s Foundation for Excellent Talents of Science, Technology & Education of Guizhou Province (No. 200817).
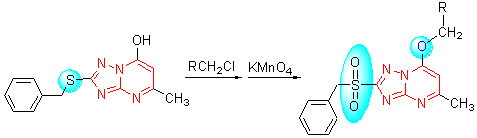
以2-苄硫基-5-甲基-7-羟基-1,2,4-三唑并[1,5-a]嘧啶为起始原料, 经过醚化、氧化反应分别合成了12个新型的2-苄硫基-5-甲基-7-取代苄氧基-1,2,4-三唑并[1,5-a]嘧啶类化合物5a~5l及其砜基类似物6a~6l, 并通过1H NMR, IR, MS和元素分析对所有目标化合物进行了结构表征. 初步生物活性测试结果表明, 在50 μg/mL浓度下, 部分化合物表现出了一定的抑菌活性, 其中化合物5c, 5k和5l对黄瓜灰霉病菌的抑制率分别为61%, 69%和85%.
关键词: 1,2,4-三唑并[1,5-a]嘧啶; 硫醚(砜); 氧醚; 合成; 抑菌活性
林选福 , 谭赞 , 刘勇 , 贺银菊 , 鲍小平 . 2-苄硫(砜)基-5-甲基-7-取代苄氧基-1,2,4-三唑并[1,5-a]嘧啶类衍生物的合成及抑菌活性研究[J]. 有机化学, 2013 , 33(02) : 353 -358 . DOI: 10.6023/cjoc201208033
Using 2-benzylthio-5-methyl-7-hydroxyl-1,2,4-triazolo[1,5-a]pyrimidine as starting material, twelve novel 2-benzylthio-5-methyl-7-substituted benzyloxy-1,2,4-triazolo[1,5-a]pyrimidine derivatives 5a~5l and their sulfonyl analogues 6a~6l were synthesized through sequential reactions of etherification and oxidation, respectively. Their structures were characterized by 1H NMR, IR, MS and elemental analysis. The preliminary bioassay indicated that some compounds exhibited certain fungicidal activities, for example, the inhibition rates of compounds 5c, 5k and 5l (50 μg/mL) against Botrytis cinerea were 61%, 69% and 85%, respectively.

/
| 〈 |
|
〉 |