

四个天然异戊烯基黄酮的全合成研究
收稿日期: 2012-10-13
修回日期: 2012-10-31
网络出版日期: 2012-11-02
基金资助
国家自然科学基金(Nos. 21162021, 20962016)、宁夏自然科学基金(No. NZ1006)、教育部“新世纪优秀人才支持计划资助”(No. NCET-09-0860)、国家重点基础研究发展计划(No. 2010CB534916)资助项目.
Study on Total Synthesis of Four Natural Prenylated Flavonoids
Received date: 2012-10-13
Revised date: 2012-10-31
Online published: 2012-11-02
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21162021, 20962016), the Ningxia Natural Science Foundation (No. NZ1006), the Program for New Century Excellent Talents in University (No. NCET-09-0860) and the National Basic Research Program 973 of China (No. 2010CB534916).
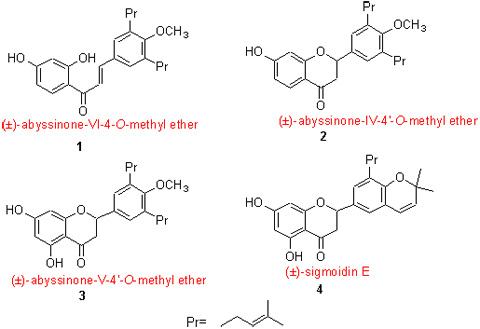
利用温和的方法进行了异戊烯基黄酮(±)-abyssinone-VI-4-O-methyl ether, (±)-abyssinone-IV-4'-O-methyl ether, (±)-abyssinone-V-4'-O-methyl ether和(±)-sigmoidin E的全合成研究, 同时通过对羟基苯甲醛的异戊烯基化以及在石油醚中低温结晶的方法合成了关键中间体4-羟基-3,5-二异戊烯基苯甲醛. 所有新化合物的结构都经过IR, 1H NMR, MS, HRMS确认.
左武标 , 杨金会 , 李红俊 , 郭冬冬 , 落俊山 , 黄文倩 . 四个天然异戊烯基黄酮的全合成研究[J]. 有机化学, 2012 , 32(12) : 2276 -2282 . DOI: 10.6023/cjoc201210016
A facile approach for the total synthesis of prenylated flavonoids, (?)-abyssinone-VI-4-O-methyl ether (1), (?)-abyssinone-IV-4'-O-methyl ether (2), (?)-abyssinone-V-4'-O-methyl ether (3) and (?)-sigmoidin E (4), has been described. The key intermediate 4-hydroxy-3,5-di-(3-methylbut-2-enyl)benzaldehyde (6) was synthesized that features regioselective prenylation of 4-hydroxybenzaldehyde and crystallizing with petroleum ether from the reaction mixture by freeze-out effect. All structures of new compounds were confirmed by IR, 1H NMR, MS and HRMS techniques.

[1] Salvatore, M. J.; Kng, A. B.; Graham A. C.; Onishi, H. R.; Bartizal, K. F.; Abruzzo, G. K.; Gill, C. J.; Ramjit, H. G.; Pitzenberger, S. M.; Witherup, K. M. J. Nat. Prod. 1998, 61, 640.
[2] Rahman, M. M.; Gray, A. I.; Khondkar, P.; Sarker, S. D. Pharm. Biol. 2008, 46, 356.
[3] Meragelman, T. L.; Tucker, K. D.; McClord, T. G.; Cardel-lina, J. H.; Shoemker, R. H. J. Nat. Prod. 2005, 68, 1790.
[4] Hirpara, K. V.; Aggarwal, P.; Mukherjee, A. J.; Joshi, N.; Burman, A. C. Curr. Med. Chem. 2009, 9, 138.
[5] Seshadri, T. R. Tetrahedron 1959, 6, 169.
[6] Yan, X.; Liu, H. Q.; Zou, Y. Q.; Ren, Z. H. Chin. J. Org. Chem. 2008, 28, 1534 (in Chinese).(延玺, 刘会青, 邹永青, 任占华, 有机化学, 2008, 28, 1534.)
[7] Oliver-Bever, B. Medicinal Plants in Tropical West Africa, Cambridge University Press, New York, 1981, p. 100.
[8] Na, M.; Jang, J.; Njamen, D.; Mbafor, J. T.; Fomum, Z. T.; Kim, B. Y.; Oh, W. K.; Ahn, J. S. J. Nat. Prod. 2006, 69, 1572.
[9] Moriyasu, M.; Ichimaru, M.; Nishiyama, Y.; Kato, A.; Mathenge, S. G; Juma, F. D.; Nganga, J. N. J. Nat. Prod. 1998, 61, 185.
[10] Yenesew, A.; Midiwo, J. O.; Miessner, M.; Heydenreich, M.; Peter, M. G. Phytochemistry 1998, 48, 1439.
[11] Promsattha, R.; Tempesta, M. S.; Fomum, Z. T.; Mbafor, J. T. J. Nat. Prod. 1988, 51, 611.
[12] Zhang, Y. H.; Yang, J. H.; Li, H. J.; Jiang, S. Z.; Li, Y. F.; Liu, W. Y. Chin. J. Chem. 2011, 29, 521.
[13] Yang, J. H.; Li, H. J.; Zhang, Y. H.; Jiang, S. Z.; Li, Y. F.; Xue, P.; Ma, Y. L.; Liu, W. Y. Chin. J. Org. Chem. 2011, 31, 1230 (in Chinese).(杨金会, 李红俊, 张玉恒, 江世智, 李云峰, 薛屏, 马玉龙, 刘万毅, 有机化学, 2011, 31, 1230.)
[14] Yang, J. H.; Luo, J. S.; Guo, D. D.; Huang, W. Q. Chin. J. Org. Chem. 2012, 32, 1749 (in Chinese).(杨金会, 落俊山, 郭冬冬, 黄文倩, 有机化学, 2012, 32, 1749.)
[15] Farmer, R. L.; Biddle, M. M.; Nibbs, A. E.; Huang, X. K.; Bergan, R. C.; Scheidt, K. A. ACS Med. Chem. Lett. 2010, 1, 400.
[16] Kazuaki, k.; Katsuo, H.; Sadakazu, Y.; Teruya, S.; Ichiro, T. S. Agric. Biol. Chem. 1975, 39, 133.
/
| 〈 |
|
〉 |