

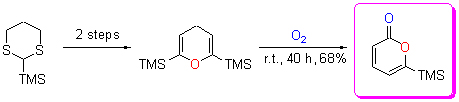
氧气氧化2,6-二-(三甲基硅基)吡喃为6-三甲基硅基-α-吡喃酮
收稿日期: 2012-09-19
修回日期: 2012-10-24
网络出版日期: 2012-11-15
基金资助
河南理工大学博士启动基金(No. 648536)资助项目.
Synthesis of 6-Trimethylsilyl-α-pyrone from 2,6-Bis(trimethylsilyl)- pyran via Mild Aerobic Oxidation
Received date: 2012-09-19
Revised date: 2012-10-24
Online published: 2012-11-15
Supported by
Project supported by the Doctoral Fund of Henan Polytechnic University (No. 648536).
开发出一种合成6-三甲基硅基α-吡喃酮的有效方法. 在无溶剂无任何催化剂的氧气气氛中, 室温条件下温和地将2,6-二-(三甲基硅基)吡喃通过氧化反应转化成6-三甲基硅基α-吡喃酮, 并且给出了该反应可能的机理.
关键词: 6-三甲基硅基α-吡喃酮; 氧化反应; 机理
周德军 , Matsuya Yuji . 氧气氧化2,6-二-(三甲基硅基)吡喃为6-三甲基硅基-α-吡喃酮[J]. 有机化学, 2013 , 33(02) : 375 -377 . DOI: 10.6023/cjoc201209027
In this study, an effective and mild method for the synthesis of 6-trimethylsilyl-α-pyrone was successfully developed. 6-Trimethylsilyl-α-pyrone, a very important intermediate in organic synthesis, was mildly synthesized from pyrane derivative via an aerobic oxidation at room temperature, without any solvents and catalysts. Furthermore, the possible mechanism of the reaction was proposed.

Key words: 6-trimethylsilyl-α-pyrone; oxidation; mechanism
[1] Tang, H.-Y.; Zeng, Y.; Li, Y.-Y.; Chen, J.-P.; Li, Y. Acta Chim. Sinica 2011, 69, 2241 (in Chinese). (唐海云, 曾毅, 李迎迎, 陈金平, 李嫕, 化学学报, 2011, 69, 2241.)
[2] Deng, Y.; Balunas, M. J.; Kim, J. A.; Lantvit, D. D.; Chin, Y. W.; Chin, H.; Sugiarso, S.; Kardono, L. B. S.; Fong, H. H. S.; Penzzuto, J. M.; Swanson, S. M.; Blanco, E. J. C.; Kinghorm, A. D. J. Nat. Prod. 2009, 72, 1165.
[3] Wang, H.; Wang, Y.; Wang, W.; Fu, P.; Liu, P.; Zhu, W. M. J. Nat. Prod. 2011, 74, 2014.
[4] Breda, S.; Reva, I.; Lapinski, L.; Cristiano, M. L. S.; Fausto, R. J. Phys. Chem. A 2006, 110, 6415.
[5] Sunazuka, T.; Omura, S. Chem. Rev. 2005, 105, 4559.
[6] Romero, D. L.; Manninen, P. R.; Han, F.; Romero, A. G. J. Org. Chem. 1999, 64, 4980.
[7] Tosaki, S. Y.; Nemoto, T.; Ohshima, T.; Shibasaki, M. Org. Lett. 2003, 5, 495.
[8] Peng, D.-Q.; Liu, Y.; Lv, Z.-F.; Xu, J.-H. Chin. J. Org. Chem. 2009, 29, 716 (in Chinese). (彭大权, 刘蕴, 吕志锋, 徐建华, 有机化学, 2009, 29, 716.)
[9] Cho, C. G.; Kim, Y. W.; Lim, Y. K.; Park, J. S.; Lee, H.; Koo, S. J. Org. Chem. 2002, 67, 290.
[10] Lanari, D.; Ballini, R.; Palmieri, A.; Pizzo, F.; Vaccaro, L. Eur. J. Org. Chem. 2011, 2874.
[11] Ohkata, K.; Lee, Y. G.; Utsumi, Y.; Ishimaru, K.; Akiba, K. Y. J. Org. Chem. 1991, 56, 5052.
[12] Perkin, W. H. J. Chem. Soc. 1868, 21, 181-186.
[13] Holden, M. S.; Crouch, R. D. J. Chem. Educ. 1998, 75, 1631.
[14] Larock, R. C.; Han, X. J.; Doty, M. J. Tetrahedron Lett. 1998, 39, 5713.
[15] Kotora, M.; Ishikawa, M.; Tsai, F. Y.; Takahashi, T. Tetrahedron 1999, 55, 4969
[16] Larock, R. C.; Doty, M. J.; Han X. J. J Org. Chem. 1999, 64, 8770.
[17] Chatadaj, W.; Corbet, M.; Furstnerm, A. Angew. Chem., Int. Ed. 2012, 51, 6929.
[18] Tsuda, T.; Morikawa, S.; Hasegawa, N.; Saegusa, T. J Org. Chem., 1990, 55, 2978.
[19] Huang, Q.; Campo, M. A.; Yao, T.; Tian, Q.; Larock, R. C. J. Org. Chem. 2004, 69, 8251.
[20] Yao, T.; Campo, M. A.; Larock, R. C. Org. Lett. 2004, 6, 2677.
[21] Saleur, D.; Bouillon, J. P.; Portella, C.; Hoffmann, N. Tetrahedron Lett. 2000, 41, 5199.
[22] Bouillon, J. P.; Portella, C. Eur. J. Org. Chem. 1999, 1571.
[23] Chuang, T. H.; Fang, J. M.; Jiaang, W. T.; Tsai, Y. M. J. Org. Chem. 1996, 61, 1794.
/
| 〈 |
|
〉 |