

4-(1H)-喹诺酮及甲氧基和溴代衍生物的合成研究
收稿日期: 2012-10-17
修回日期: 2012-11-05
网络出版日期: 2012-11-15
基金资助
国家自然科学基金(No. B020601);四川省自然科学重点基金(No. 07ZA109)资助项目.
Syntheses of 4-(1H)-Quinolones and the Brominated or Methoxy Substituted Derivatives
Received date: 2012-10-17
Revised date: 2012-11-05
Online published: 2012-11-15
Supported by
Project supported by the National Natural Science Foundation of China (No. B020601), the Natural Science Foundation of Sichuan Province (No. 07ZA109).
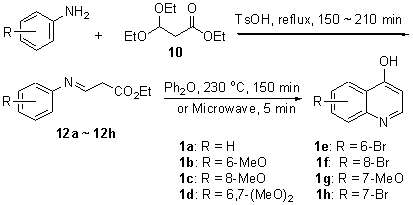
提出了一种4-(1H)-喹诺酮衍生物合成方法. 以3,3-二乙氧基丙酸乙酯和苯胺为原料, 生成中间体3-(苯基亚氨基)丙酸乙酯, 再经热环化或微波促进环化两步反应制备4-(1H)-喹诺酮, 并以此方法合成了7个溴代或甲氧基取代的4-(1H)-喹诺酮衍生物.
关键词: 4-(1H)-喹诺酮; 3,3-二乙氧基丙酸乙酯; 合成方法; 环化
马梦林 , 黄剑彪 , 王玉良 , 陈淑华 , 陈华 . 4-(1H)-喹诺酮及甲氧基和溴代衍生物的合成研究[J]. 有机化学, 2013 , 33(02) : 378 -382 . DOI: 10.6023/cjoc201210031
A simple and new synthetic approach to 4-(1H)-quinolones was described. This method was started from ethyl 3,3-diethoxypropanoate and aniline via a synthetic sequence of preparation of key intermediate 3-(phenylimino)propionate and cyclization reaction. The brominated or methoxy substituted derivatives were synthesized by this new method as well.

/
| 〈 |
|
〉 |