

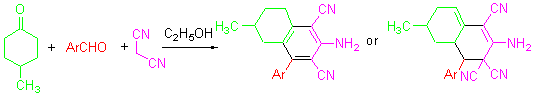
方便、有效地合成2-氨基-4-芳基-6-甲基四氢化萘衍生物
收稿日期: 2012-10-12
修回日期: 2012-11-21
网络出版日期: 2012-11-26
基金资助
江苏省高校自然科学基础研究基金(No. 08KJB150017)、江苏师范大学科研平台(No. 10XLS02)和国家自然科学基金委员会创新研究群体科学基金 (No. 50921002)资助项目.
A Facile and Efficient Synthesis of 2-Amino-6-methyl-4-aryltetrahydronaphthalene Derivatives
Received date: 2012-10-12
Revised date: 2012-11-21
Online published: 2012-11-26
Supported by
Project supported by the Natural Science Foundation of Jiangsu Education Department (No. 08KJB150017), the Foundation of Xuzhou Normal University (No. 10XLS02), and the Foundation from National Natural Science Foundation of China for Innovative Research Group (No. 50921002).
荣良策 , 魏贤勇 , 宗志敏 , 韩笑 , 张诚义 , 沈小沣 , 陈翔 . 方便、有效地合成2-氨基-4-芳基-6-甲基四氢化萘衍生物[J]. 有机化学, 2013 , 33(03) : 648 -651 . DOI: 10.6023/cjoc201210014
A facile and efficient synthesis of novel 2-amino-6-methyl-4-aryltetrahydronaphthalene derivatives by the reaction of aromatic aldehydes, 4-methylcyclohexanone, and malononitrile in the presence of NaOH and 95% EtOH is reported. This protocol has the advantages of shorter reaction time, mild conditions, and easy work-up. The products were identified by 1H NMR, IR and HRMS techniques. The reported method is the efficient approach for the synthesis of these compounds.
/
| 〈 |
|
〉 |