

功能化石墨烯负载冬凌草甲素抗肿瘤制剂的研究
收稿日期: 2012-11-16
修回日期: 2012-12-01
网络出版日期: 2012-12-07
基金资助
国家自然科学基金(Nos. 31100549, 21204098)、上海市青年科技启明星跟踪计划(No. 11QH1402800)和上海市生物医药科技重点(No. 10431903000)资助项目.
Functionalized Graphene Oxide as a Nanocarrier for Loading and Delivering of Oridonin
Received date: 2012-11-16
Revised date: 2012-12-01
Online published: 2012-12-07
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 31100549, 21204098), the Shanghai Rising Star Program (No. 11QH1402800), and the Shanghai Scientific and Technological Innovation Project (No. 10431903000).
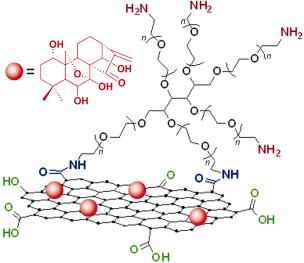
通过改进的Hummers法制备氧化石墨烯(GO), 利用酰胺化反应将端基为氨基的六臂聚乙二醇(PEG)连到氧化石墨烯表面, 改善其水溶性和生物相容性. 原子力显微镜(AFM)数据表明所制备的GO-PEG尺寸小于250 nm, 稳定性试验证明GO-PEG在水和PBS缓冲液中可以很好地分散. 利用制备的GO-PEG作为药物载体, 通过物理共混的方法负载疏水性抗肿瘤药物——冬凌草甲素. 紫外光谱法测得载药率高达105%, 远高于一般其他的药物载体. 选择肺癌细胞A549和乳腺癌细胞MCF-7对载药体系的细胞毒性进行了研究, 结果表明即使在高达100 mg/L的浓度下培养48 h, 载体GO-PEG对两种细胞仍然具有很小的毒性(相对细胞存活率>85%), 而通过载体负载后冬凌草甲素的疗效有所增强, 对细胞具有更大的杀伤作用.
徐志远 , 李永军 , 史萍 , 王博婵 , 黄晓宇 . 功能化石墨烯负载冬凌草甲素抗肿瘤制剂的研究[J]. 有机化学, 2013 , 33(03) : 573 -580 . DOI: 10.6023/cjoc201211033
Graphene oxide (GO) was prepared by modified Hummers method firstly. In order to improve its water solubility and biocompatibility, 6-armed PEG was grafted onto GO via an amidation process. The size of GO-PEG was less than 250 nm and stability test indicated excellent dispersibility of GO-PEG in water and PBS buffer. Furthermore, oridonin, a widely used cancer chemotherapy drug, is adsorbed onto GO-PEG via blending. The drug loading ratio was determined to be as high as 105%, which was much higher than other common drug carriers. A549 lung cancer cell and MCF-7 breast cancer cell were chosen to study the cytotoxicity of GO-PEG/oridonin, GO-PEG, and free oridonin. The results demonstrated that GO-PEG did not show obvious toxicity (relative cell viability>85%), even cultivated for 48 h at a relatively high concentration of 100 mg/L. Compared to oridonin, the GO-PEG/oridonin nanocarrier shows higher cytotoxicity in A549 and MCF-7 cells.

Key words: graphene oxide; oridonin; drug delivery
/
| 〈 |
|
〉 |