

新型二阶非线性光学生色团的合成及辅助给体对分子性能的影响研究
收稿日期: 2012-09-13
修回日期: 2012-12-12
网络出版日期: 2012-12-18
基金资助
国家自然科学基金(No. 60771044)资助项目
Synthesis of Novel Organic Nonlinear Optical Chromophores and the Enhancement of Electro-optic Activity of the Additional Donors
Received date: 2012-09-13
Revised date: 2012-12-12
Online published: 2012-12-18
Supported by
Project supported by the National Natural Science Foundation of China (No. 60771044).
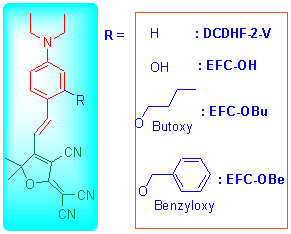
为了研究辅助给体对生色团分子热分解温度以及二阶极化率的影响, 以已报道生色团分子2-二氰亚甲基-3-氰基-4-(4-二乙胺基-苯乙烯基)-5,5-二甲基-2,5-二氢呋喃(DCDHF-2-V)为基础, 分别在苯环二乙胺基的邻位上以羟基、丁氧基和苄氧基作为辅助给体合成了三种新型的生色团分子: EFC-OH, EFC-OBu和EFC-OBe. 通过核磁共振、红外光谱以及元素分析表征确认了其结构. 热失重分析结果表明, 三种分子的热性能良好, 其中EFC-OBe的热分解温度(Td)最高为278.6 ℃. 溶致变色法测定结果表明, EFC-OBe的二阶非线性光学系数(μgβ1064 nm)最高, 为47149×10-48 esu. 将这三种分子的Td值和μgβ值与未加入辅助给体的DCDHF-2-V分子进行对比, 结果表明辅助给体的加入对分子的热稳定性能影响不大, 但能显著提高分子的μgβ值一个数量级以上.
关键词: 非线性光学; 辅助给体; 热失重分析(TGA); 溶致变色法
游英才 , 唐先忠 , 唐翔 , 贾鲲鹏 , 王洋 , 李昱树 , 张煜 . 新型二阶非线性光学生色团的合成及辅助给体对分子性能的影响研究[J]. 有机化学, 2013 , 33(03) : 530 -534 . DOI: 10.6023/cjoc201209020
In order to study the impact of different additional donors on molecular thermal decomposition temperature (Td) value of the chromophores as well as the second polarization (μgβ) value. Three novel materials EFC-OH, EFC-OBu and EFC- OBe were synthesized by attaching additional donors to the benzene ring based on the reported 2-[{4-(4-diethylamino)sty- reneyl}-3-cyano-5,5-dimethyl-5H-furan-2-ylidene]malononitrile (DCDHF-2-V). The three chromophores were characterized by 1H NMR, 13C NHR, FT-IR and elemental analysis. The Td value was determined by TGA testing, and the result showed that three molecules had a good thermal performance. EFC-OBe processing a Td of 278.6 ℃ showed the best among these molecules. The hyperpolarizability was measured and calculated by solvatochromism method. EFC-OBe still showed the best μgβ value of 4.7149×10-44 esu. Comparing to the DCDHF-2-V without additional donors on Td value and μgβ value, additional donors had little effect on Td value while achieved an improvement of one order of magnitude in μgβ values, which indicates that the molecules can work in high temperature and performance better in optics.

/
| 〈 |
|
〉 |