

带有不同端炔保护基团的新型苯乙炔树枝状化合物的合成
Synthesis of Novel Phenylacetylene Dendrimers Containing Different Protective Groups for Their Terminal Acetylenes
Received date: 2012-11-15
Revised date: 2012-12-14
Online published: 2012-12-18
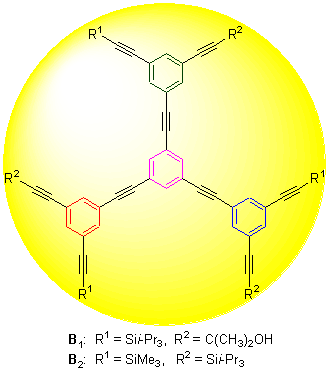
以1,3,5-三溴苯为原料, 通过Sonogashira反应, 设计并合成了两种带有不同端炔保护基团的1,3,5-取代的苯乙炔树枝状化合物: 1,3,5-三[3-(3-甲基-3-羟基-1-丁炔基)-5-(三异丙基硅乙炔基)苯基乙炔基]苯(B1)和1,3,5-三[3-(三甲基硅乙炔基)-5-(三异丙基硅乙炔基)苯基乙炔基]苯(B2), 并对合成路线的选择进行了比较和讨论. 用1H NMR, 13C NMR, 质谱, 元素分析等表征手段确认了中间体及最终产物的结构. 这两种苯乙炔树枝状化合物各自带有两类不同的周边端炔保护基团, 可根据其脱保护条件的不同引入不同的周边功能基团.
许晨 , 黄鹏程 . 带有不同端炔保护基团的新型苯乙炔树枝状化合物的合成[J]. 有机化学, 2013 , 33(03) : 551 -557 . DOI: 10.6023/cjoc201211031
Using Sonogashira coupling reaction, two kinds of 1,3,5-substituted phenylacetylene dendrimers: 1,3,5-tris[3-(3- hydroxy-3-methyl-1-butynyl)-5-(triisopropylsilylethynyl)phenylethynyl]benzene (B1) and 1,3,5-tris[3-(trimethylsilylethynyl)- 5-(triisopropylsilylethynyl)phenylethynyl]benzene (B2) were synthetized and the synthetic routes were compared and discussed. The structures of the target compounds and intermediates were confirmed by 1H NMR, 13C NMR, EI-MS, HRMS techniques and element analysis. In phenylacetylene dendrimers B1 and B2, the six terminal acetylene groups on their periphery were protected by two different kinds of groups, respectively. Based on the different deprotection conditions, two different kinds of functional groups could be introduced to B1 and B2, respectively.

/
| 〈 |
|
〉 |