

新型侧链含氟苯并噁嗪的合成及其性能的研究
收稿日期: 2012-12-11
修回日期: 2013-01-06
网络出版日期: 2012-01-09
基金资助
国家科技部重大专项资金(No. 2011ZX02703)资助项目.
Synthesis and Properties of the Polymer Based on Benzoxazine with Two Perfluorohexyl Side Chains
Received date: 2012-12-11
Revised date: 2013-01-06
Online published: 2012-01-09
Supported by
Project supported by the Ministry of Science and Technology of China (No. 2011ZX02703).
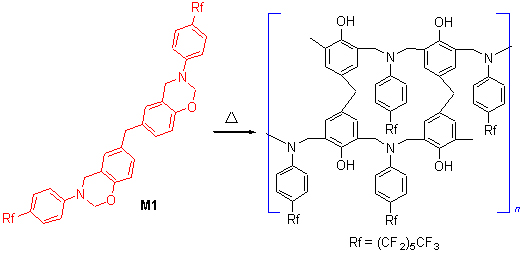
采用简洁的方法, 合成了一种新型侧链含长氟碳链的苯并噁嗪单体(M1), 并研究了其热固化反应和固化产物的性能. 结果表明, M1的起始固化温度为257 ℃, 但当加入5 wt%的咪唑催化剂后, 其固化起始温度降低至183 ℃. M1聚合物具有优异的热稳定性, 在氮气氛中其5%和10%热重损失温度分别为337和382 ℃, 800 ℃碳残余率为45.9%. 该固化树脂亦具有良好的介电性能, 在1 MHz频率下其介电常数为3.78. 常温下固化树脂的吸水率小于1%, 表面自由能为6.98 mN/m. 以上结果表明侧链含氟的苯并噁嗪有望成为一种性能优异的新型绝缘材料.
吴建平 , 赖华 , 刁屾 , 金凯凯 , 袁超 , 房强 . 新型侧链含氟苯并噁嗪的合成及其性能的研究[J]. 有机化学, 2013 , 33(05) : 1042 -1046 . DOI: 10.6023/cjoc201212013
A new benzoxazine with two perfluorohexyl side chains (M1) was synthesized and its homopolymerization was investigated. The results showed that the curing of M1 was occurred at near 257 ℃, whereas the curing onset temperature can be lowered to 183 ℃ when a catalyst of 5 wt% imidazole was added. The cured M1 exhibited higher thermal stability with 5% and 10% weight loss temperatures of 337 and 382 ℃, and a char yield of 45.9%, respectively. The cured M1 exhibited also good electrical properties with dielectric constant of 3.78 in a frequency of 1 MHz. The water absorption of the cured M1 was below 1%, and the surface free energy was 6.98 mN/m, respectively.

/
| 〈 |
|
〉 |