

2-烷氧羰基烷基/正丁基-4-芳基-1,5-苯并硫氮杂(卄卓)类化合物的合成及抑菌活性的研究
收稿日期: 2013-10-23
修回日期: 2013-01-18
网络出版日期: 2013-02-22
基金资助
国家自然科学基金(No. 20972040);河北省教育厅青年基金(No. Q2012001)和河北工业职业技术学院青年基金(No. Qz1102)资助项目
Synthesis and Antifungal Activity of 2-Carbalkoxyalkyl/(n-butyl)-4-aryl-1,5-benzothiazepines
Received date: 2013-10-23
Revised date: 2013-01-18
Online published: 2013-02-22
Supported by
Project supported by the National Natural Science Foundation of China (No. 20972040), the Research Project of Education Department of Hebei Province of China (No. Q2012001), and the Scientific Research Fund of Hebei College of Industry and Technology (No. Qz1102).
设计、合成了三类2位取代-1,5-苯并硫氮杂(卄卓)衍生物: 2-烷氧羰基甲基-4-芳基-1,5-苯并硫氮杂(卄卓)、2-烷氧羰基乙基-4-芳基-1,5-苯并硫氮杂(卄卓)和2-正丙基-4-芳基-1,5-苯并硫氮杂(卄卓). 所有目标化合物结构经过元素分析, IR, MS, HRMS及1H NMR确证.研究了2-烷氧羰基烷基/正丁基-4-芳基-1,5-苯并硫氮杂(卄卓)的合成反应条件, 测定了目标化合物的抑真菌活性. 通过对该类化合物抑真菌活性的评价, 进一步明确乙酯基在1,5-苯并硫氮杂(卄卓)1的生物活性中所起的作用.
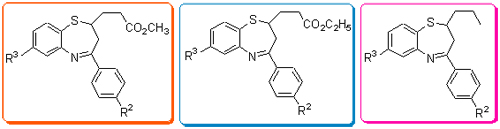
关键词: 1,5-苯并硫氮杂(卄卓); 合成; 抑真菌活性; 构效关系
李文红 , 刘喜莹 , 张博 , 李媛 . 2-烷氧羰基烷基/正丁基-4-芳基-1,5-苯并硫氮杂(卄卓)类化合物的合成及抑菌活性的研究[J]. 有机化学, 2013 , 33(07) : 1503 -1508 . DOI: 10.6023/cjoc201210044
A series of novel 2-substituted-1,5-benzothiazepines were synthesized successfully and their structures were determined by elemental analysis, IR, MS/HRMS and 1H NMR. The synthetic conditions of 2-substituted-4-aryl-1,5-benzothiazepines were investigated. The antifungal activities of these 2-substituted-1,5-benzothiazepine derivatives were tested and the results confirmed that the 2-ethyl carboxylate was necessary for antifungal activity in 1,5-benzothiazepine compounds 1.

/
| 〈 |
|
〉 |