

红紫素-18二酰亚胺的化学反应及其叶绿素类二氢卟吩衍生物的合成
收稿日期: 2013-01-18
修回日期: 2013-03-01
网络出版日期: 2013-03-15
基金资助
国家自然科学基金(No. 21272048)和山东省黄金工程技术研究中心(2011年度)资助项目.
Chemical Reaction of Purpurin-18 Imide and Synthesis of Chlorins Related to Chlorophyll
Received date: 2013-01-18
Revised date: 2013-03-01
Online published: 2013-03-15
Supported by
Project supported by the National Natural Science Foundations of China (No. 21272048) and the Project of Shandong Applied Reaearch Centre of Gold Nanotechnology (2011).
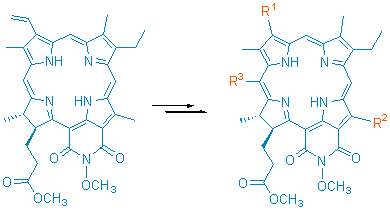
以N-甲氧基红紫素-18二酰亚胺甲酯为起始原料, 利用其二氢卟吩大环上的活性反应区域, 分别进行了3-位乙烯基的亲电加成和1,3-偶极环加成反应、20-meso-位的亲电取代反应、12-位甲基的空气氧化和亲核取代反应的研究, 在红紫素-18二酰亚胺周环上的C(3)-, C(12)-和C(20)-meso-位上构建和引进不同的化学结构和取代基团, 完成了11个未见报道的叶绿素类二氢卟吩衍生物的合成, 其化学结构均经UV, 1H NMR, IR及元素分析予以证实; 对相应的化学反应也提出了可能的反应机理.
关键词: 叶绿素-a; 红紫素-18 二酰亚胺; 二氢卟吩; 化学反应; 合成
纪建业 , 夏尚文 , 赵丽丽 , 李家柱 , 祁彩霞 , 王进军 . 红紫素-18二酰亚胺的化学反应及其叶绿素类二氢卟吩衍生物的合成[J]. 有机化学, 2013 , 33(07) : 1457 -1464 . DOI: 10.6023/cjoc201301044
Purpurin-18 imide methyl ester was used as a starting material, and its chemical reactions, including the electrophilic addition and 1,3-polar cycloaddition on the vinyl group at 3-position, the electrophilic substitution at 20-meso-position, the allomerization of C(12)-methyl group and nucleophilic substitution, were carried out making use of the chemical reactivities on the chlorin macrocycle. The different chemical structures and substituent groups were established and introduced at C(3)-, C(12)-and C(20)-meso-potation on the periphery of purpurin-18 imide. The synthesis of 11 unreported chlorins related to chlorophyll was accomplished and their chemical structures were characterized by UV, 1H NMR, IR spectra and elemental analysis. The possible mechanisms about corresponding reactions were tentatively proposed.

Key words: chlorophyll-a; purpurin-18 imide; chlorin; chemical reaction; synthesis
/
| 〈 |
|
〉 |