

(2S,3aR,7aS)-八氢-1H-吲哚-2-甲酸苄酯的合成
收稿日期: 2013-02-02
修回日期: 2013-02-21
网络出版日期: 2013-02-22
基金资助
国家自然科学基金(Nos. 21172143, 21172145, 21232004)资助项目.
Synthesis of (2S,3aR,7aS)-Benzyl Octahydro-1H-indole-2-carboxylate
Received date: 2013-02-02
Revised date: 2013-02-21
Online published: 2013-02-22
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21172143, 21172145, 21232004).
通过合成路线的改进以及合成工艺的优化, 方便高效地得到了抗高血压药物群多普利的关键中间体 (2S,3aR,7aS)-八氢-1H-吲哚-2-甲酸苄酯. 产品结构及绝对构型通过NMR及HPLC进行表征, 与文献报道的数据完全一致. 最终以99%的对映选择性及13.2%的产率得到目标化合物. 为群多普利关键中间体的合成提供了一条可工业化的途径.
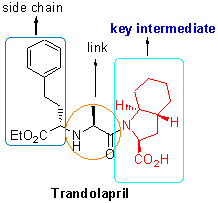
关键词: 群多普利; 八氢-1H-吲哚-2-甲酸苄酯; 合成
时涛 , 申杰峰 , 安前进 , 刘德龙 , 刘燕刚 , 张万斌 . (2S,3aR,7aS)-八氢-1H-吲哚-2-甲酸苄酯的合成[J]. 有机化学, 2013 , 33(07) : 1573 -1577 . DOI: 10.6023/cjoc201302006
The key intermediate of antihypertensive drugs trandolapril, (2S,3aR,7aS)-benzyl octahydro-1H-indole-2-car-boxylate, was synthesized by improvement of synthetic route and optimation of synthetic technology with satisfactory results (99% ee and 13.2% overall yield). The structure and absolute configuration of the product were characterized by NMR and HPLC analysis, which are completely consistent with the reported data of the literature. It was obvious that the current methodology provided an efficient pathway for the synthesis of trandolapril.

/
| 〈 |
|
〉 |