

铜绿假单胞菌PAO1III型分泌系统新型抑制剂的合成和生物活性研究
收稿日期: 2013-02-26
修回日期: 2013-04-01
网络出版日期: 2013-04-08
基金资助
国家自然科学基金(No.21272029)和江苏省“333高层次人才培养工程”资助项目.
Synthesis and Bioactivity of Novel Inhibitors for Type III Secretion System of Pseudomonas aeruginosa PAO1
Received date: 2013-02-26
Revised date: 2013-04-01
Online published: 2013-04-08
Supported by
Project supported by the National Natural Science Foundation of China (No.21272029) and the "333 High-level talent training program" of Jiangsu Province.
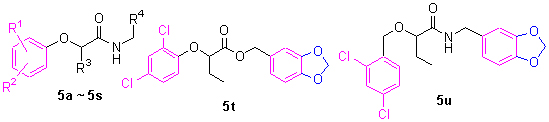
铜绿假单胞菌PAO1是一种革兰氏阴性机会性人类病原菌, 易感染免疫受损的人群.III型分泌系统(Type three secretion system, T3SS)是其主要的致病因子.T3SS抑制剂的策略是抑制铜绿假单胞菌表达及分泌毒力蛋白, 阻止其对宿主细胞的侵染.在一种已知T3SS抑制剂的结构基础上, 设计和合成了20种α-苯氧基酰胺新衍生物, 系统研究了它们的构效关系, 研究表明有5种新衍生物对铜绿假单胞菌的一个效应子编码基因exoS的表达具有明显抑制作用.其中N-(2-吡啶基甲基)-2-(2,4-二氯苯氧)-丁酰胺(5r)的活性强于已知的抑制剂MBX1641, 并具有很好的水溶性.
张成芳 , 吴小刚 , 李燕 , 梁翠荣 , 车亦舟 , 顾玲玲 , 任杰 , 胡昆 , 孙小强 , Ching-Hong Yang , 陈新 . 铜绿假单胞菌PAO1III型分泌系统新型抑制剂的合成和生物活性研究[J]. 有机化学, 2013 , 33(06) : 1309 -1318 . DOI: 10.6023/cjoc201302021
Pseudomonas aeruginosa PAO1 is a Gram-negative, opportunistic bacterial human pathogen which infects immunocompromised individuals.The bacterium carries a type III secretion system (T3SS) as a major virulence determinant.The strategy of T3SS inhibitors is to prevent the bacterium from injecting effector proteins into the host, and causing a change in the pathophysiology of the host cells.Based on the structure of a known T3SS inhibitor of P.aeruginosa, 20 new α-phenoxyacetamide derivatives have been designed and synthesized, and the structure-activity relationship results for these new derivatives have been discussed.Five derivatives have shown strong inhibitory effect against exoS gene expression of P.aeruginosa, and among them, N-(2-pyridylmethyl)-2-(2,4-dichlorophenoxy)-butanamide (5r) has not only exhibited stronger potency than the known T3SS inhibitor, but also better solubility in aqueous solution.

Key words: Pseudomonas aeruginosa; type III secretion system; inhibitors; synthesis
/
| 〈 |
|
〉 |