

岗稔根五环三萜类化学成分的研究
收稿日期: 2013-03-19
修回日期: 2013-04-11
网络出版日期: 2013-04-17
基金资助
国家自然科学基金(Nos.81273401, 81202420)、教育部高等学校博士学科点(博导类)基金(No.20120071110049)和“博士点新教师基金”(No.20120071120049)以及科技部“重大新药创制”重大专项公共资源平台(No.2011ZX09307-002-01)资助项目.
Pentacyclic Triterpenoids from the Roots of Rhodomyrtus tomentosa
Received date: 2013-03-19
Revised date: 2013-04-11
Online published: 2013-04-17
Supported by
Project supported by the National Natural Science Foundation of China (Nos.81273401, 81202420), the Ph.D.Programs Foundation of Ministry of Education of China (Nos.20120071110049, 20120071120049) and the Grant of Ministry of Science and Technology (MOST, No.2011ZX09307-002-01).
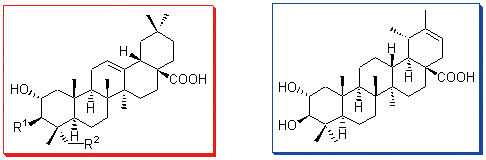
首次对桃金娘Rhodomyrtus tomentosa根部(岗稔根)的化学成分展开研究, 从其甲醇提取物中分离得到8个五环三萜类化合物.上述化合物的结构经一维、二维核磁共振谱和质谱等波谱技术分别鉴定为: 23-O-顺式-对-香豆酰基- 2α,3β-二羟基齐墩果烷-12-烯-28-酸(1), 23-O-反式-对-香豆酰基-2α,3β-二羟基齐墩果烷-12-烯-28-酸(2), 3β-O-反式-阿魏酰基-2α,23-二羟基齐墩果烷-12-烯-28-酸(3), 3β-O-反式-对-香豆酰基-2α,23-二羟基齐墩果烷-12-烯-28-酸(4), 3β-O-顺 式-对-香豆酰基-2α,23-二羟基齐墩果烷-12-烯-28-酸(5), 山楂酸(6), 阿江揽仁酸(7)和2α,3β-dihydroxytaraxer-20- en-28-oic acid (8).其中化合物1和3为新的齐墩果烷型三萜, 化合物2, 4~6和8为首次从该植物中分离得到.
熊娟 , 黄亚 , 唐宇 , 尤梅 , 胡金锋 . 岗稔根五环三萜类化学成分的研究[J]. 有机化学, 2013 , 33(06) : 1304 -1308 . DOI: 10.6023/cjoc201303029
Eight pentacyclic triterpenoids were isolated from the methanol extract of the roots of Rhodomyrtus tomentosa.By application of spectroscopic methods (especially 1D-, 2D-NMR, MS), the structures were established as 23-cis-p-coumaroyl- oxy-2α,3β-dihydroxyolean-12-en-28-oic acid (1), 23-trans-p-coumaroyloxy-2α,3β-dihydroxyolean-12-en-28-oic acid (2), 3β- O-trans-ferulyl-2α,23-dihydroxyolean-12-en-28-oic acid (3), 3β-O-trans-p-coumaroyl-2α,23-dihydroxyolean-12-en-28-oic acid (4), 3β-O-cis-p-coumaroyl-2α,23-dihydroxyolean-12-en-28-oic acid (5), maslinic acid (6), arjunolic acid (7), and 2α,3β-dihydroxytaraxer-20-en-28-oic acid (8).Among them, compounds 1 and 3 were new oleanane-type triterpenoids, while compounds 2, 4~6 and 8 were isolated from this plant for the first time.This is the first report on the chemical constituents from the roots of R.tomentosa.

/
| 〈 |
|
〉 |