

3-(2-氯-4-三氟甲基苯氧基)苯甲酸酯的合成、晶体结构及生物活性
收稿日期: 2013-02-25
修回日期: 2013-05-04
网络出版日期: 2013-05-08
基金资助
国家重点基础研究发展规划(973计划, No. 2010CB126100);国家自然科学基金(Nos. 21002037, 21172090);教育部创新团队(PCSIRT, No. IRT0953)资助项目
Synthesis, Crystal Structure and Biological Activity of 3-(2-Chloro-4-trifluoromethylphenoxy)benzoate
Received date: 2013-02-25
Revised date: 2013-05-04
Online published: 2013-05-08
Supported by
Project supported by the National Basic Research Program of China ((973 Program, No. 2010CB126100)), the National Natural Science Foundation of China (Nos. 21002037, 21172090) and the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, No. IRT0953)
彭浩 , 高玉焦 , 贺红武 . 3-(2-氯-4-三氟甲基苯氧基)苯甲酸酯的合成、晶体结构及生物活性[J]. 有机化学, 2013 , 33(9) : 1994 -1998 . DOI: 10.6023/cjoc201301060
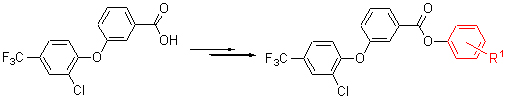
A series of new 3-(2-chloro-4-trifluoromethylphenoxy)benzoates (2) have been synthesized by the reactions of substituted phenols with intermediate 3-(2-chloro-4-trifluoromethylphenoxy)benzoyl chloride (1). The structures of compounds have been confirmed by 1H NMR, IR, EI-MS and elemental analyses. The structure of 3-methylphenyl 3-(2-chloro-4-trifluoromethylphenoxy)benzoate (2f) has been determined by single crystal X-ray diffraction. The results of preliminary bioassay indicated that most of title compounds possess a moderate herbicidal activity against dicotyledonous weeds.
Key words: synthesis; diphenyl ether; herbicidal activity
/
| 〈 |
|
〉 |