

对甲苯磺酸催化的2-乙酰氧甲基吡咯衍生物和醇的醚化反应研究
收稿日期: 2013-03-01
修回日期: 2013-04-28
网络出版日期: 2013-05-08
基金资助
江西省自然科学基金(No. 2009GZH0078)资助项目
Etherification of 2-Acetoxymethylpyrrole Derivative with Alcohol Catalyzed by para-Toluenesulfonic Acid
Received date: 2013-03-01
Revised date: 2013-04-28
Online published: 2013-05-08
Supported by
Project supported by the Natural Science Foundation of Jiangxi Province (No. 2009GZH0078)
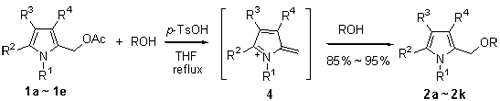
研究了对甲苯磺酸催化的2-乙酰氧甲基吡咯衍生物和醇之间的醚化反应, 结果以优秀的收率生成了相应的2-烷氧甲基吡咯衍生物. 该反应具有条件温和、产物得率高和后处理简单等优点, 提供了一种在弱酸条件下制备2-烷氧甲基吡咯衍生物的新方法, 提出了一种基于氮杂富勒烯过渡态的反应机理用于解释实验现象.
关键词: 2-乙酰氧甲基吡咯衍生物; 2-烷氧甲基吡咯衍生物; 醚化反应; 氮杂富勒烯; 醇
严兆华 , 余信权 , 刘永杰 , 许云 , 林森 . 对甲苯磺酸催化的2-乙酰氧甲基吡咯衍生物和醇的醚化反应研究[J]. 有机化学, 2013 , 33(9) : 1975 -1981 . DOI: 10.6023/cjoc201302018
Etherification of 2-acetoxymethylpyrrole derivatives with alcohols catalyzed by para-toluenesulfonic acid was investigated in details resulting in the smooth formation of the corresponding 2-alkoxymethylpyrrole derivatives in excellent yields. An alternate method for the preparation of 2-alkoxymethylpyrrole derivatives starting from 2-acetoxymethylpyrrole derivatives under weak acidic condition was provided. The main advantages of the developed methodology are mild reaction conditions, high yields of products and simple work-up procedure. A plausible mechanism involving the generation of highly reactive azafulvene intermediate 4 was proposed to explain the observed phenomena.

/
| 〈 |
|
〉 |