

N-芳基-4-(吡啶-2-基)-5-(1H-1,2,4-三唑-1-基)噻唑-2-胺衍生物的合成及生物活性研究
收稿日期: 2013-04-09
修回日期: 2013-05-20
网络出版日期: 2013-05-31
Synthesis and Biological Evaluation of N-Phenyl-4-(pyridin-2-yl)-5-(1H-1,2,4-triazol-1-yl)thiazol-2-amines
Received date: 2013-04-09
Revised date: 2013-05-20
Online published: 2013-05-31
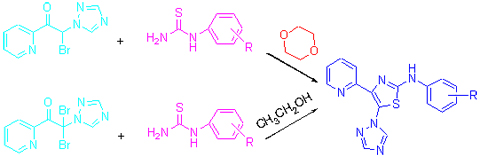
利用Hantzsch反应合成了13个N-芳基-4-(吡啶-2-基)-5-(1H-1,2,4-三唑-1-基)噻唑-2-胺衍生物, 所有化合物的结构均经1H NMR, MS及元素分析确证. 研究发现, 溶剂对不同取代基的目标化合物的合成影响很大. 生物活性测试结果表明, 化合物均具有一定的杀菌活性. 其中, 化合物6c对番茄早疫和苹果轮纹、化合物6g对番茄早疫、化合物6h对黄瓜黑腥及化合物6i对苹果轮纹均显示80%以上的杀菌活性.
关键词: 2-氨基噻唑; 1H-1,2,4-三唑; 合成; 生物活性
张沐 , 罗喜爱 , 祝岱帧 , 张雪鹏 , 刘凤 , 方建新 , 刘建兵 . N-芳基-4-(吡啶-2-基)-5-(1H-1,2,4-三唑-1-基)噻唑-2-胺衍生物的合成及生物活性研究[J]. 有机化学, 2013 , 33(9) : 1955 -1959 . DOI: 10.6023/cjoc201304010
Thirteen new N-phenyl-4-(pyridin-2-yl)-5-(1H-1,2,4-triazol-1-yl)thiazol-2-amines were synthesized using Hantzsch reaction. Their structures of all these new compounds have been confirmed by 1H NMR, MS and elemental analysis. It is found that the solvent used in the reaction has important influence on the synthesis of the title compounds containing different substituent. Their results of bioassay showed that all title compounds exhibited some degree of antifungal activities. Compound 6c against A. solani and P. piricola, compound 6g against A. solani, compound 6h against C. cucumerinum and compound 6i against P. piricola displayed above 80% inhibition, respectively.

Key words: 2-aminothiazole; 1H-1,2,4-triazole; synthesis; biological activities
/
| 〈 |
|
〉 |