

N-亚苄基橙皮素腙及类似物的合成与细胞活性
收稿日期: 2013-04-18
修回日期: 2013-05-22
网络出版日期: 2013-05-31
基金资助
国家自然科学基金(No. 81060261);广西科学自然科学基金重点(No. 2011jjD20002)资助项目
Synthesis and Cytotoxicity of N-Benzylidene Hesperitin Hydrazones and Analogues
Received date: 2013-04-18
Revised date: 2013-05-22
Online published: 2013-05-31
Supported by
Project supported by the National Natural Science Foundation of China (No. 81060261) and the Natural Science Foundation of Guangxi Province (No. 2011jjD20002)
刘志平 , 韦万兴 , 甘春芳 , 黄燕敏 , 刘盛 , 周敏 , 崔建国 . N-亚苄基橙皮素腙及类似物的合成与细胞活性[J]. 有机化学, 2013 , 33(9) : 1988 -1993 . DOI: 10.6023/cjoc201304028
Hesperitin hydrazone (2) was generated by the reaction of natural hesperitin (1) as raw material with hydrazine hydrate. Eight N-benzylidene hesperitin hydrazones 3~10 and six analogues 11~16 were synthesized by the reaction of 2 with various aldehydes, respectively. The structures of all reaction products were confirmed by NMR, IR and HR-MS techniques. And the cytotoxity against human cancer cell SGC-7901 of the synthesized compounds was also evaluated. The results showed 5, 7, 10 and 16 exhibiting distinct cytotoxity against SGC-7901.
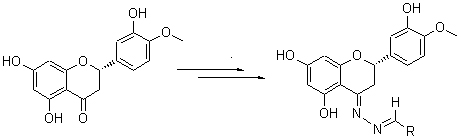
Key words: hesperitin; hesperitin hydrazones; N-benzylidene; cytotoxicity
/
| 〈 |
|
〉 |