

新型含咪唑啉2,4-二酮的磷酰胺类化合物的合成和生物活性
收稿日期: 2013-05-14
修回日期: 2013-06-11
网络出版日期: 2013-06-21
基金资助
“十二五”科技支撑计划(No. 2011BAE06B03);国家自然科学基金(No. 20772150)和南开大学元素有机化学国家重点实验室开放基金(Nos. 0902, 201003)资助项目
Synthesis and Biological Activity of Novel Phosphoramide with Hydantoin
Received date: 2013-05-14
Revised date: 2013-06-11
Online published: 2013-06-21
Supported by
Project supported by the National Key Technologies R&D Program (No. 2011BAE06B03), the National Natural Science Foundation of China (No. 20772150) and the National Key Laboratory of Elemento-Organic Chemistry in Nankai University (No. 0902, 201003).
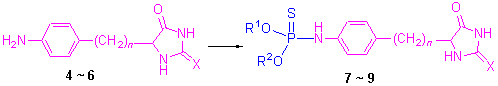
在合成含咪唑啉2,4-二酮磷酸酯和磷酰胺酯类化合物的基础上, 将5-[4-氨基-苯(苄)基]-2,4-咪唑啉二酮中间体与O,O-二烷(芳)基硫代磷酰氯反应合成得到了38个结构新颖的O,O-二烷(芳)基-5-[4-氨基-苯(苄)基]-2,4-咪唑啉二酮硫代磷酰胺类化合物, 其结构通过1H NMR, 31P NMR, IR, 元素分析和X射线衍射表征. 化合物9b: C14H20N3O3PS2· 1/3H2O, Mr=379.43, 三斜晶系, P-1空间群, a=0.82092(5) nm, b=1.79810(14) nm, c=2.0153(2) nm, α=72.289(8)°, β=79.355(7)°, γ=77.288(6)°, V=2.7421(4) nm3, Dc=1.379 g/cm3, Z=6, F(000)=1196, μ(Mo Kα)=0.397 mm-1, S=1.031, final R=0.0426, wR=0.0797. 初步生物测定结果表明化合物O,O-二甲基-5-(4-氨基苯基)-2,4-咪唑啉二酮硫代磷酰胺(7a), O-乙基-O-苯基-5-(4-氨基苄基)-2-硫代-2,4-咪唑啉二酮硫代磷酰胺(9n)和O,O-二甲基-5-(3-氨基苯基)-2-硫代-2,4-咪唑啉二酮硫代磷酰胺(11a)在100 μg/mL浓度下对油菜的抑制率分别为75.4%, 80.5%, 81.7%, 其中11个化合物对蚜虫的LD50 值在182.41~368.52 μg/mL之间, 表现了良好的杀虫活性.
关键词: 咪唑啉-2,4-二酮; 硫代磷酰胺; 腺苷酸琥珀酸合成酶; 生物活性
王进敏 , 徐志红 , 韩金涛 , 董宏波 , 刘斌 , 王明安 . 新型含咪唑啉2,4-二酮的磷酰胺类化合物的合成和生物活性[J]. 有机化学, 2013 , 33(10) : 2186 -2195 . DOI: 10.6023/cjoc201305023
Based on the synthesis of phosphates and phosphorylamidates with hydantoin, thirty and eight new O,O-dialkyl-(aryl)-5-[4-aminophenyl(benzyl)]-2,4-imidazolidinedione thiophosphoramide derivatives with hydantoin were synthesized by the reaction of O,O-dialkyl(aryl) thiophosphoryl chloride with 5-(4-aminophenyl)-and 5-(4-aminobenzyl)-2,4-imidazolidine-dione intermediates, and their structures were confirmed by 1H NMR, 31P NMR, IR, elemental analysis and X-ray diffraction. 9b: C14H20N3O3PS2·1/3H2O, Mr=379.43, triclinic, space group P-1, a=0.82092(5) nm, b=1.79810(14) nm, c=2.0153(2) nm, α=72.289(8)°, β=79.355(7)°, γ=77.288(6)°, V=2.7421(4) nm3, Dc=1.379 g/cm3, Z=6, F(000)=1196, μ(Mo Kα)=0.397 mm-1, S=1.031, final R=0.0426, wR=0.0797. The preliminary bioassay showed that O,O-dimethyl-5-(4-amino-phenyl)-2,4-imidazolidinedione thiophosphoramide (7a), O-ethyl-O-phenyl-5-(4-aminobenzyl)-2,4-imidazolidine-dione thio-phosphoramide (9n) and O,O-dimethyl-5-(3-aminophenyl)-2-thio-2,4-imidazolidinedione thiophosphoramide (11a)have inhibitory rates 75.4%, 80.5% and 81.7% against Brasica campestris at the concentration of 100 μg/mL, while eleven compounds showed excellent insecticidal activities against Myzus Persicae with the LD50 data in the range of 182.41~368.52 μg/mL, respectively.

/
| 〈 |
|
〉 |