

咪唑型离子液负载手性双膦配体pyrphos的合成及不对称氢化研究
收稿日期: 2013-05-30
修回日期: 2013-07-06
网络出版日期: 2013-07-10
基金资助
国家自然科学基金(Nos. 21172064, 20972045);湖南省自然科学基金(No. 10JJ2006)和湖南省教育厅重点(No. 10A022)资助项目
Study on Synthesis of Imidazolium-Tagged Chiral Pyrphos Diphosphine Ligands and Their Applications in Asymmetric Hydrogenation
Received date: 2013-05-30
Revised date: 2013-07-06
Online published: 2013-07-10
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21172064, 20972045), the Natural Science Foundation of Hunan Province (No.10JJ2006), and the Key Scientific Research Fund of Hunan Provincial Education Department (No. 10A022).
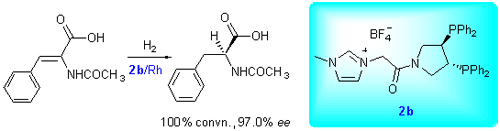
采用N-甲基咪唑类离子液体为载体, 以C2轴手性的双膦配体pyrphos[(R,R)-3,4-bis(diphenylphosphino)pyr-rolidine]为嫁接对象, 研究了N-甲基咪唑型离子液体负载手性双膦配体pyrphos的合成方法, 确定了目标分子的合成路线及提纯方法, 对目标化合物和相关中间体进行了1H NMR, 13C NMR, 31P NMR, IR和HRMS等表征. 研究了该类配体与金属铑组成的催化剂在脱氢氨基酸的不对称氢化反应中的应用, 结果表明: 采用[bmim]BF4/MeOH混合溶剂, 以N-甲基咪唑四氟合硼酸盐离子液负载的pyrphos膦配体与[Rh(COD)2]BF4形成的配合物为催化剂, 在α-乙酰氨基肉桂酸的不对称氢化反应中获得了与小分子催化剂相似的催化性能, 产物的转化率为100%, 对映选择性为97.0%. 催化剂循环使用4次, 活性和对映选择性基本不变.
易兵 , 武高峰 , 周威 , 何华平 . 咪唑型离子液负载手性双膦配体pyrphos的合成及不对称氢化研究[J]. 有机化学, 2013 , 33(10) : 2143 -2147 . DOI: 10.6023/cjoc201305042
Herein we developed a facile method for preparation of a new functionalized ionic liquids supported chiral diphosphine ligands by grafting homogeneous chiral pyrphos[(R,R)-3,4-bis(diphenylphosphino)pyrrolidine] ligand with C2 axis onto imidazolium ionic liquids supports. Firstly the linker groups having halogen atoms were attached to amino group in pyrphos, and then reacted with N-methylimidazole. The other anion-containing chiral ligands located on ionic liqids (ILs) were synthesized via ion exchange technique. These ligands and the related intermediates were purified by flash column chromatography and were characterized by 1H NMR, 13C NMR, 31P NMR, IR and HRMS techniques. All results were consistent with the compounds synthesized. Then we focused on the catalytic performances and the feasibility of catalyst recycling in rhodium-catalyzed asymmetric hydrogenation of prechiral dehydroamino acid. The results showed that the catalyst generated in situ by imidazolium-tagged pyrphos ligands with BF4 as counteranion and[Rh(COD)2]BF4 exhibited high catalytic activity and enantioselectivity in asymmetric hydrogenation of α-acetamido cinnamic acid in[bmim]BF4/MeOH cosolvent systems, which was similar to the molecule catalyst. The conversion is 100%, and the ee value is up to 97.0%. Moreover, the catalyst could be reused four times without significant loss of activity and enantioselectivity.

/
| 〈 |
|
〉 |