

2-乙氧羰基(羧基)-4-取代苯基-2,3-二氢-1H-苯并[b][1,4]二氮杂(艹卓)的合成及抑菌活性测定
收稿日期: 2013-05-14
修回日期: 2013-06-07
网络出版日期: 2013-07-11
基金资助
国家自然科学基金(No. 21276064)、河北省教育厅自然科学基金(No. 2008320)和河北师大校内基金(No. 2011Y04)资助项目.
An Efficient Catalytic Synthesis and Antimicrobial Activities of Derivatives of 2-Ethoxycarbonyl(carboxyl)-4-substituted phenyl-2,3-dihydro-1H-benzo[b][1,4]diazepine
Received date: 2013-05-14
Revised date: 2013-06-07
Online published: 2013-07-11
Supported by
Project supported by the National Natural Science Foundation of China (No. 21276064), the Natural Science Foundation of Hebei Education Department (No. 2008320), and the Science Foundation of Hebei Normal University (No. 2011Y04).
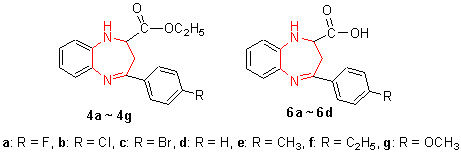
以取代苯和顺丁烯二酸酐为起始原料,通过Friedel-Crafts酰基化反应、酯化、aza-Michael加成、环合、脱水等过程,合成了11种未见文献报道的2-乙氧羰基(羧基)-4-取代苯基-2,3-二氢-1H-苯并[b][1,4]二氮杂(艹卓)(4a~4g和6a~6d)化合物. 通过1H NMR,IR,MS和元素分析确定了目标产物的结构,对合成目标化合物的反应条件、选择性进行了较详细的研究,提出了可能的反应机理. 对新合成的目标化合物进行了体外抑菌活性测试,结果表明,部分化合物显示了一定的抑菌活性,并表现出对不同菌株的抑菌活性具有一定的选择性和特异性. 采用密度泛函理论方法在B3LYP/6-31G水平上对所合成的11种目标化合物进行了几何全优化和计算,研究其微观结构和抑菌活性之间的关系,结果表明,N(11)和C=N可能分别是所合成的该类化合物与细菌(枯草杆菌)和真菌(白色念珠菌和新型隐球菌)发生作用的主要活性部位.
王兰芝 , 花中霞 , 王莎莎 . 2-乙氧羰基(羧基)-4-取代苯基-2,3-二氢-1H-苯并[b][1,4]二氮杂(艹卓)的合成及抑菌活性测定[J]. 有机化学, 2013 , 33(11) : 2376 -2383 . DOI: 10.6023/cjoc201305022
Seven novel ethoxycarboxyl-containing 2-ethoxycarbonyl-4-substituted phenyl-2, 3-dihydro-1H-benzo[b][1, 4]dia-zepines 4a~4g and four novel carboxyl-containing 2-carboxyl-4-substituted phenyl-2, 3-dihydro-1H-benzo[b]-[1, 4]diazepines 6a~6d were efficiently synthesized by the Friedel-Crafts acylation reaction, aza-Michael addition reaction, esterification, dehydration cyclization reaction, where different substituted benzene and maleic anhydride were used for the raw materials. The structures of these new compounds were determined by 1H NMR, IR, FAB mass spectral analysis and elemental analysis. The exhaustive researches on the synthetic reaction of the class of compounds were carried out and the reaction mechanism has been unequivocally established in this paper. Furthermore, newly synthesized compounds were evaluated for their antimicrobial activity. The result showed that most of the title compounds are biologically active and have obvious specificity and selective antibacterial activities to different classificatory bacterium. Density functional theory (DFT) B3LYP with 6-31G basis set has been used to investigate target compounds. Inferred N (11) and the group of C=N were active sites of inhibition bacterial (B. subtilis) and fungal (C. albicans and C. neofonmans) respectively.

/
| 〈 |
|
〉 |