

红紫素-18的周环结构修饰及其叶绿素类二氢卟吩衍生物的合成
收稿日期: 2013-05-03
修回日期: 2013-07-14
网络出版日期: 2013-07-19
基金资助
国家自然科学基金(No. 21272048)和山东省黄金工程技术研究中心(2011年度)资助项目.
Modifications for Peripheral Structures of Purpurin-18 and Synthesis of Chlorophyllous Chlorin Derivatives
Received date: 2013-05-03
Revised date: 2013-07-14
Online published: 2013-07-19
Supported by
Project supported by the National Natural Science Foundations of China (No. 21272048) and the Project of Shandong Applied Reaearch Centre of Gold Nano-technology (2011).
梁波颖 , 刘洋 , 徐希森 , 金英学 , 祁彩霞 , 王进军 . 红紫素-18的周环结构修饰及其叶绿素类二氢卟吩衍生物的合成[J]. 有机化学, 2013 , 33(11) : 2357 -2366 . DOI: 10.6023/cjoc201305006
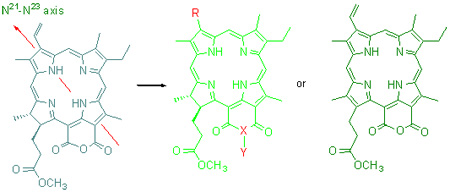
Purpurin-18 methyl ester was used as a starting material, and the modifications for the structure on the terminal of its N21-N23-axis were completed making use of electron-richous properties of C (3)-vinyl group by various reactions including oxidation, reduction, electrophilic addition and 1, 3-polar cycloaddition. The substituted groups, which could conjugated with macrocyclic chromophore in different form, were introduce to 3-position on the periphery, respectively. The synthesis of 11 unreported chlorophyllous chlorins with basic skeloton of purpurin-18 was accomplished and their chemical structures were characterized by elemental analysis, UV, IR and 1H NMR spectra. The influence of peripheric structure of purpurin-18 on their electronic spectrum was discussed and the possible mechanisms about corresponding reactions were tentatively proposed.
Key words: chlorophyll-a; chlorin; purpurin-18; chemical modification; synthesis
/
| 〈 |
|
〉 |