

酰胺官能团构建方法研究新进展
收稿日期: 2013-05-29
修回日期: 2013-07-02
网络出版日期: 2013-07-26
基金资助
山东省自然科学基金(No. ZR2012CL15)资助项目.
New Advances of the Methods of Amide Function Group for Construction
Received date: 2013-05-29
Revised date: 2013-07-02
Online published: 2013-07-26
Supported by
Project supported by the Natural Science Foundation of Shandong Province (No. ZR2012CL15).
盛国柱 , 张炜 . 酰胺官能团构建方法研究新进展[J]. 有机化学, 2013 , 33(11) : 2271 -2282 . DOI: 10.6023/cjoc201305048
Amides are one of the most ubiquitous and important functional groups in natural products and synthetic drugs. The research of forming methods for amide is of great significance, also it is now a hotspot in the research of the synthetic method. Herein, the synthetic methods of amides in recent years are reviewed, which is of great significance for future research.
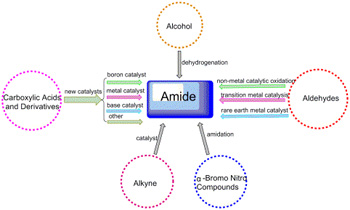
Key words: amide; synthetic methods; research progress
[1] Ishihara,K.; Ohara,S.; Yamamoto,H.J.Org.Chem.1996,61,4196.
[2] Nguyen,T.B.; Sorres,J.; Tran,M.Q.; Ermolenko,L.; Al-Mourabit,A.Org.Lett.2012,14,3202.
[3] Charville,H.; Jackson,D.; Hodges,G.; Whiting,A.Chem.Commun.2010,46,1813.
[4] Starkov.P,Sheppard.T.D.Org.Biomol.Chem.2011,9,1320.
[5] Mali,S.M.; Jadhav,S.V.; Gopi,H.N.Chem.Commun.2012,48,7085.
[6] Li,M.; Hu,L.; Cao,X.Q.; Hong,H.Y.; Lu,J.M.; Gu,H.W.Chem.Eur.J.2011,17,2763.
[7] Ghaffarzadeh,M.; Joghan,S.S.; Faraji,F.Tetrahedron Lett.2012,53,203.
[8] Zhu,C.L.; Zou,X.F.J.Zhejiang Normal Univ.(Nat.Sci.) 2004,27,261 (in Chinese).
(祝春兰,邹雪飞,浙江师范大学学报(自然科学版),2004,27,261.)
[9] Shi,F.; Li,J.; Li,C.J.; Jia,X.S.Tetrahedron Lett.2010,51,6049.
[10] Cho,D.H.; Jang,D.O.Tetrahedron Lett.2004,45,2285.
[11] Ohshima,T.; Hayashi,Y.; Agura,K.; FujⅡ,Y.; Yoshiyama,A.; Mashima,K.Chem.Commun.2012,48,5434.
[12] Wahba,A.E.; Peng,J.N.; Hamann,M.T.Tetrahedron.Lett.2009,50,3901.
[13] Li,G.; Wang,X.W.; Tian,C.; Zhang,T.B.; Zhang,Z.L.; Liu,J.Y.Tetrahedron Lett.2012,53,5193.
[14] Bhattacharya,A.; Suarez,V.; Jr.,V.T.; Wu,J.J.Tetrahedron Lett.2006,47,3221.
[15] Comerford,J.W.; Clark,J.H.; Macquarrie,D.G.; Breeden,S.W.Chem.Commun.2009,18,2562.
[16] Markó,I.E.; Mekhalfia,A.Tetrahedron Lett.1990,31,7237.
[17] Liu,Z.J.; Zhang,J.; Chen,S.L.Angew.Chem.,Int.Ed.2012,51,3231.
[18] Kekeli E.K.; Wolf,C.Org.Lett.2007,9,3429.
[19] Prasad,V.; Kale,R.R.; Mishra,B.B.; Kumar,D.; Tiwari,V.K.Org.Lett.2012,14,2936.
[20] Wang,X.B.; David; Wang,Z.G.Tetrahedron 2011,67,3406.
[21] Shaw,A.Y.; Denning,C.R.; Hulme,C.Tetrahedron Lett.2012,53,4151.
[22] Ramesh,K.; Murthy,S.N.; Karnakar,K.; Reddy,K.H.V.; Nageswar,Y.V.D.; Vijay,M.; Devi,B.P.; Prasad,R.Tetrahedron Lett.2012,53,2636.
[23] Colombeau,L.; Traoré,T.; Compain P.; Martin,O.R.J.Org.Chem.2008,73,8647.
[24] Tamaru,Y.; Yamada,Y.; Yoshida,Z.Synthesis 1983,474.
[25] Suto,Y.; Yamagiwa,N.; Torisawa,Y.Tetrahedron Lett.2008,49,5732.
[26] Ghosh,S.C.; Ngiam,J.S.Y.; Chai,C.L.L.; Seayad,A.M.; Dang,T.T.; Chen,A.Q.Adv.Synth.Catal.2012,354,1407.
[27] Yoo,W.J.; Li,C.J.J.Am.Chem.Soc.2006,128,13064.
[28] Ghosh,S.C.; Ngiam,J.S.Y.; Seayad,A.M.; Tuan,D.T.; Chai,C.L.L.; Chen,A.Q.J.Org.Chem.2012,77,8007.
[29] Zhang,C.; Xu,Z.J.; Zhang L.R.; Jiao,N.Angew.Chem.,Int.Ed.2011,50,11088.
[30] Zhang,C.; Zong,X.L.; Zhang,L.R.; Jiao,N.Org.Lett.2012,14,3280.
[31] Zhu,M.W.; Fujita,K.; Yamaguchi,R.J.Org.Chem.2012,77,9102.
[32] Cadoni,R.; Porcheddu,A.; Giacomelli,G.; Luca,L.D.Org.Lett.2012,14,5014.
[33] Chang,J.W.W.; Ton,T.M.U.; Tania,S.; Taylor,P.C.; Chan,P.W.H.Chem.Commun.2010,46,922.
[34] Qin,Y.C.; Peng,Q.; Song,J.S.; Zhou,D.Tetrahedron Lett.2011,52,5880.
[35] Gai,L.L.,Kung K.K.Y.; Wong,M.K.Chem.Commun.2012,48,4112.
[36] Allam,B.K.; Singh,K.N.Tetrahedron Lett.2011,52,5851.
[37] Zhang,L.J.; Wang,S.W.; Zhou,S.L.; Yang,G.S.; Sheng,E.H.J.Org.Chem.2006,71,3149.
[38] Seo,S.Y.; Marks,T.J.Org.Lett.2008,10,317.
[39] Thomson,J.A.; Schafer,L.L.Dalton Trans.2012,7897.
[40] Qian,C.W.; Zhang,X.M.; Li,J.M.; Xu,F.; Zhang,Y.; Shen,Q.Organometallics 2009,28,3856.
[41] Li,J.M.; Xu,F.; Zhang,Y.; Shen,Q.J.Org.Chem.2009,74,2575.
[42] CAI C.Y.; Li,L.; Xu,F.; Shen,Q.Chin.Sci.Bull.2010,55,3641.
[43] Xu,B.; Huang,L.L.; Yang,Z.J.; Yao,Y.M.; Zhang,Y.; Shen,Q.Organometallics 2011,30,3588.
[44] Xu,K.; Hu,Y.B.; Zhang,S.; Zha,Z.G.; Wang,Z.Y.Chem.Eur.J.2012,18,9793.
[45] Naota,T.; Murahashi,S.I.Synlett 1991,693.
[46] Nordstrøm,L.U.; Vogt,H.; Madsen,R.J.Am.Chem.Soc.2008,130,17672.
[47] Ghosh,S.C.; Muthaiah,S.; Zhang,Y.; Xu,X.Y.; Hong,S.H.Adv.Synth.Catal.2009,351,2643.
[48] Zhang,Y.; Chen,C.; Ghosh,S.C.; Li,Y.X.; Hong,S.H.Organometallics 2010,29,1374.
[49] Maki,B.E.; Scheidt,K.A.Org.Lett.2009,11,1651.
[50] Gunanathan,C.; Ben-David,Y.; Milstein,D.Science 2007,317,790.
[51] Zhu,J.L.; Zhang,Y.; Shi,F.; Deng,Y.Q.Tetrahedron Lett.2012,53,3178.
[52] Chen C.; Hong,S.H.Org.Lett.2012,14,2992.
[53] Xiao,F.H.; Liu,Y.; Tang,C.L.; Deng,G.J.Org.Lett.2012,14,984.
[54] Das,R.; Chakraborty,D.Catal.Commun.2012,26,48.
[55] Chan,W.K.; Ho,C.M.; Wong,M.K.; Che,C.M.J.Am.Chem.Soc.2006,128,14796.
[56] Cho,S.H.; Yoo,E.J.; Bae,I.; Chang,S.J.Am.Chem.Soc.2005,127,16046.
[57] Wei,W.; Hu,X.Y.; Yan,X.W.; Zhang,Q.; Cheng,M.; Ji,J.X.Chem.Commun.2012,48,305.
[58] Shen,B.; Makley,D.M.; Johnston,J.N.Nature 2010,465,1027.
/
| 〈 |
|
〉 |