

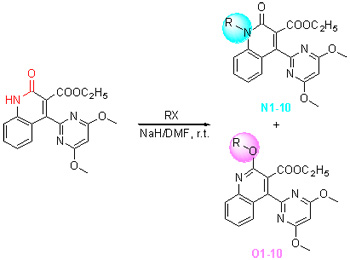
4-(4,6-二甲氧基嘧啶-2-基)-2-氧代-1,2-二氢喹啉-3-羧酸乙酯的烃化反应
收稿日期: 2013-06-04
修回日期: 2013-07-18
网络出版日期: 2013-07-26
基金资助
湖南省科技计划(No. 2012NK3098)、湖南省教育厅科学研究(No. 11A092)、怀化市科技计划(No. 2013)资助项目
N-Alkylation and O-Alkylation of Ethyl 4-(4,6-Dimethoxy-pyrimidin-2-yl)-2-oxo-1,2-dihydroquinoline-3-carboxylate
Received date: 2013-06-04
Revised date: 2013-07-18
Online published: 2013-07-26
Supported by
Project supported by the Planned Science and Technology Project of Hunan Province (No. 2012NK3098), the Scientific Research Fund of Hunan Provincial Education Department (No. 11A092) and the Planned Science and Technology Project of Huaihua City (No. 2013).
李元祥 . 4-(4,6-二甲氧基嘧啶-2-基)-2-氧代-1,2-二氢喹啉-3-羧酸乙酯的烃化反应[J]. 有机化学, 2013 , 33(11) : 2334 -2340 . DOI: 10.6023/cjoc201306004
Twenty novel N-substitued and O-substitued compounds were prepared starting from ethyl 4-(4, 6-dimethoxy-py-rimidin-2-yl)-2-oxo-1, 2-dihydroquinoline-3-carboxylate. Various halogenated hydrocarbons were used as resource of alkylation and NaH as base in N, N-dimethylformamide (DMF) at room temperature. The structures of alkylation products were confirmed by 1H NMR, IR, MS and elemental analysis. Two isomer structures were further determined by representative X-ray structures of N10 and O10. The alkylation reaction was performed in mild conditions with a total yield of 89%~98%. Among them, the main product N-Alkylation was obtained in 47%~77% yield, and O-Alkylation was simultaneously obtained in 21%~49% yield by a simple one-pot reaction.
Key words: quinolinone; alkylation; structural characterization
[1] (a) Khalid,E.S.; Mansour,S.A.S.; Farouk,S.E.F.; Samir,A.R.J.Nat.Prod.2000,63,995.
(b) He,J.; Lion,U.; Sattler,I.; Gollmick,F.A.; Grabley,S.; Cai,J.M.; Meiners,M.; SchÜnke,H.; Schaumann,K.; Dechert,U.; Krohn,M.J.Nat.Prod.2005,68,1397.
[2] (a) Payne,J.E.; Bonnefous,C.; Symons,K.T.; Nguyen,P.M.; Sablad,M.; Rozenkrants,N.; Zhang,Y.; Wang,L.; Yazdani,N.; Shiau,A.K.; Noble,S.A.; Rix,P.; Rao,T.S.; Hassig,C.A.; Smith,N.D.J.Med.Chem.2010,53,7739.
(b) Liu,Q.S.; Chang,J.W.; Wang,J.H.; Kang,S.A.; Thoreen,C.C.; Markhard,A.; Hur,W.; Zhang,J.M.; Sim,T.; Sabatini,D.M.; Gray,N.S.J.Med.Chem.2010,53,7146.
(c) Lucas,S.; Negri,M.; Heim,R.; Zimmer,C.; Hartmann,R.W.J.Med.Chem.2011,54,2307.
(d) Yin,L.; Lucas,S.; Maurer,F.; Kazmaier,U.; Hu,Q.Z.; Hartmann,R.W.J.Med.Chem.2012,55,6629.
[3] Vrudhula,V.M.; Dasgupta,B.; Qian-Cutrone,J.; Kozlowski,E.S.; Boissard,C.G.; Dworetzky,S.I.; Wu,D.; Gao,Q.; Kimura,R.; Gribkoff,V.K.; Starrett,J.E.J.Med.Chem.2007,50,1050.
[4] Freeman,G.A.; C.Andrews,W.; Hopkins,A.L.; Lowell,G.S.; Schaller,L.T.; Cowan,J.R.; Gonzales,S.S.; Koszalka,G.W.; Hazen,R.J.; Boone,L.R.; Ferris,R.G.; Creech,K.L.; Roberts,G.B.; Short,S.A.; Weaver,K.; Reynolds,D.J.; Milton,J.; Ren,J.; Stuart,D.I.; Stammers,D.K.; Chan,J.H.J.Med.Chem.2004,47,5923.
[5] Anerson,R.J.; Cloudsdale,L.S.; Hokama,T.EP 0461079,1991 [Chem.Abstr.1991,116,128972].
[6] (a) Detsi,A.; Bouloumbasi,D.; Prousis,K.C.; Koufaki,M.; Athanasellis,G.; Melagraki,G.; Afantitis,A.; Igglessi-Markopoulou,O.; Kontogiorgis.C.; Hadjipavlou-Litina,D.J.J.Med.Chem.2007,50,2450.
(b) Ryckebusch,A.; Garcin,D.; Lansiaux,A.; Goossens,J.F.; Baldeyrou,B.; Houssin,R.; Bailly,C.; Hénichart,J.P.J.Med.Chem.2008,51,3617.
[7] Marzano,C.; Chilin,A.; Baccichetti,F.; Bettio,F.Eur.J.Med.Chem.2004,39,411.
[8] (a) Ukrainets,I.V.; Sidorenko,L.V.; Gorokhova,O.V.; Bereznyakova,N,L.; Shishkina,S.V.Chem.Heterocycl.Compd.2006,42,1296.
(b) Ukrainets,I.V.; Bereznyakova,N.L.; Parshikov,V.A.; Turov,A.V.Chem.Heterocycl.Comp.2007,43,1269.
[9] Ali,I.A.I.; Fathalla,W.; Rayes,S.M.E.ARKIVOC 2008,13,179.
[10] Ahvale,A.B.; Prokopcová.H.; Šef?ovi?ová,J.; Steinschifter.W.; Täubl,A.E.; Uray.G.; Stadlbauer,W.Eur.J.Org.Chem.2008,563.
[11] Woo,L.W.L.; Ganeshapillai,D.; Thomas,M.P.; Sutcliffe,O.B.; Malini,B.; Mahon,M.F.; Purohit,A.; Potter,B.V.L.ChemMedChem 2011,6,2019.
[12] (a) Priya,N.; Gupta,A.; Chand,K.; Singh,P.; Kathuria,A.; Raj,H.G.; Parmar,V.S.; Sharma,S.K.Bioorg.Med.Chem.2010,18,4085.
(b) Chilin,A.; Marzano,C.; Baccichetti,F.; Simonato,M.; Guiotto,A.Bioorg.Med.Chem.2003,11,1311.
(c) Park,K.K.; Lee,J.J.Tetrahedron.2004,60,2993.
[13] Peifer,C.; Urich,R.; Schattel,V.; Abadleh,M.; Röttig,M.; Kohlbacher,O.; Laufer,S.Bioorg.Med.Chem.Lett.2008,18,1431.
[14] (a) Merabet,N.; Dumond,J.; Collinet,B.; Baelinghem,L.V.; Boggetto,N.; Ongeri,S.; Ressad,F.; Reboud-Ravaux,M.; Sicsic,S.J.Med.Chem.2004,47,6392.
(b) Guo,Z.X.; Cainmldge,A.N.; McKⅡlop,A.; Horwell,D.C.Tetrahedron Lett.1999,40,6999.
(c) Geneste,H.; Backfisch,G.; Braje,W.; Delzer,J.; Haupt,A.; Hutchins,C.W.; King,L.L.; Lubisch,W.; Steiner,G.; Teschendorf,H.J.; Ungera,L.; Wernet,W.Bioorg.Med.Chem.Lett.2006,16,658.
[15] Li,Y.X.; Yan,X.H.; Hu,W.Y.Spec.Petrochem.2011,28(5),57 (in Chinese).(李元祥,晏小红,胡蔚昱,精细石油化工,2011,28(5),57.)
/
| 〈 |
|
〉 |