

4,5-二取代的菲和9,10-二氢菲衍生物的合成及液晶性能研究
收稿日期: 2013-06-20
修回日期: 2013-07-15
网络出版日期: 2013-07-26
基金资助
国家自然科学基金(No. 21032004)、高等学校博士学科点专项科研基金(No. 20110002110051)和国家电子和信息产业发展基金资助项目.
Synthesis and Liquid Crystal Property of 4,5-Disubstituted Phenanthrene and 9,10-Dihydrophenanthrene Derivatives
Received date: 2013-06-20
Revised date: 2013-07-15
Online published: 2013-07-26
Supported by
Project supported by the National Natural Science Foundation of China (No. 21032004), the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20110002110051) and the National Electronics and Information Industry Development Fund.
曹建华 , 李敏 , 隋岩 , 华瑞茂 . 4,5-二取代的菲和9,10-二氢菲衍生物的合成及液晶性能研究[J]. 有机化学, 2013 , 33(11) : 2349 -2356 . DOI: 10.6023/cjoc201306029
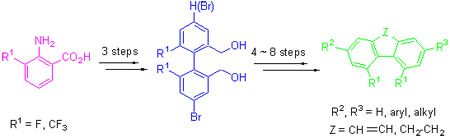
Using 2-amino-3-substituted benzoic acid as starting material, 4, 5-disubstituted phenanthrene derivatives were synthesized via sequential reactions of diazotization-coupling reaction, esterification, reduction, oxidation and cyclization, and using reduced biphenyl intermediates as starting materials. 4, 5-Disubstituted 9, 10-dihydrophenanthrene derivatives were syn-thesized via sequential reactions of bromination, grinard reaction, using 4, 5-disubstituted phenanthrene and 4, 5-disubstituted 9, 10-dihydrophenanthrene derivatives as starting materials. Liquid crystal monomer compounds were synthesized via sequential reactions of halogenations and coupling reaction, and the chemical structures of products were identified by 1H NMR, 13C NMR spectra.
[1] Xu,H.Ph.D.Dissertation,Jilin University,Changchun,2009 (in Chinese).(许海,博士论文,吉林大学,长春,2009.)
[2] Dubois,F.; Gingras,M.Tetrahedron Lett.1998,39,5039.
[3] Kharasch,M.S.; Nudenberg,W.; Fields,E.K.J.Am.Chem.Soc.1944,66,1276.
[4] Campeau,L.C.; Parisien,M.; Jean,A.; Fagnou,K.J.Am.Chem.Soc.2006,128,581.
[5] Brown,C.; Sikkel,B.J.; Carvalho,C.F.; Sargent,M.V.J.Chem.Soc.,Perk.Trans.1 1982,3007.
[6] Shi,M.; Xu,B.J.Org.Chem.2002,67,294.
[7] Kuninobu,Y.; Tatsuzaki,T.; Matsuki,T.; Takai,K.J.Org.Chem.2011,76,7005.
[8] Some,S.; Dutta,B.; Ray,J.K.Tetrahedron Lett.2006,47,1221.
[9] Bacon,L.J.Chem.Soc.1958,1375.
[10] Cosmo,R.; Sternhell,S.Aust.J.Chem.1987,40,35.
[11] Yosuke,N.; Takahiro,F.; Sachiko,S.; Jun,N.Chem.Lett.1999,10,1039.
[12] Tian,H.; Shi,J.; Dong,S.; Yan,D.; Wang,L.-X.; Geng,Y.; Wang,F.S.Chem.Commun.2006,33,3498.
[13] Cosmo,R.; Sternhell,S.Australian J.Chem.1987,40,2137.
[14] Witting,G.; Zimmermann,H.Chem.Ber.1953,86,629.
[15] Newman,M.S.; Lilje,K.C.J.Org.Chem.1979,44,4944.
[16] Ramurthy,S.; Lin,X.; Subramanian,S.; Rico,A.C.; Wang,X.M.; Jain,R.; Murray,J.M.; Bashman,S.E.; Warne,R.L.; Shu,W.; Zhou,Y.; Dove,J.; Aikawa,M.; Amiri,P.; Wang,W.; Jensen,J.M.; Wagman,A.S.; Pfister,K.B.; Ng,S.C.WO 2007117607,2007 [Chem.Abstr.2007,147,469365].
[17] Ligtenbarg,A.G.J.; Beuken,E.K.; Meetsma,A.; Veldman,N.; Smeets,W.J.J.J.Chem.Soc.1998,2,263.
[18] Dameron,A.A.; Ciszek,J.W.; Tour,J.M.; Weisc,P.S.J.Phys.Chem.B 2004,108,16761.
[19] Ciszek,J.W.; Tour,J.M.Tetrahedron Lett.2004,45,2801.
[20] Kawamura,M.; Funahashi,M.US 20070029927,2007 [Chem.Abstr.2007,146,216108].
[21] Helms,A.; Heiler,D.; Mclendon,G.J.Am.Chem.Soc.1992,114,6227.
[22] Vonlanthen,D.; Rotzler,J.; Neuburger,M.; Mayor,M.Eur.J.Org.Chem.2010,75,120.
/
| 〈 |
|
〉 |