

无溶剂下三氟甲磺酸及稀土盐催化氟苯的傅克酰基化反应的研究
收稿日期: 2013-05-30
修回日期: 2013-07-21
网络出版日期: 2013-08-12
基金资助
国家重点基础研究发展计划(973计划,No. 2010CB732300)资助项目.
Solvent-Free Friedel-Crafts Acylation of Fluorobenzene Catalyzed by Trifluoromethanesulfonic Acid and Rare Earth Triflates
Received date: 2013-05-30
Revised date: 2013-07-21
Online published: 2013-08-12
Supported by
Project supported by the National Basic Research Program (973 Program, No. 2010CB732300).
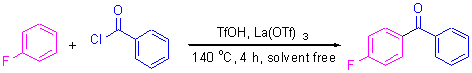
研究了在无溶剂条件下以催化剂量的三氟甲磺酸(TfOH)及稀土盐(Re(OTf)3)为复合催化剂,对氟苯与苯甲酰氯的傅克酰基化反应的催化性能. 研究发现,由于少量固体酸Re(OTf)3的添加,可以使液体酸催化剂的用量减少一半,且TfOH和Re(OTf)3间存在协同作用. 对于氟苯与苯甲酰氯的傅克酰基化反应,考察了11种稀土Re(OTf)3的作用,发现La(OTf)3具有最好的助催化活性,且可重复使用,经140 ℃反应4 h后,产物对氟二苯甲酮的选择性高于99%,产率达到87%. 对于不同反应物的酰基化反应,不同稀土的Re(OTf)3显示出不同的助催化作用. 对于二甲苯的傅克酰基化反应,在所测试的Re(OTf)3催化剂中,Ce(OTf)3的助催化效果最显著.
贾红燕 , 谢作松 , 郭耘 , 郭杨龙 , 王艳芹 , 卢冠忠 . 无溶剂下三氟甲磺酸及稀土盐催化氟苯的傅克酰基化反应的研究[J]. 有机化学, 2013 , 33(12) : 2572 -2577 . DOI: 10.6023/cjoc201305049
Solvent-free Friedel-Crafts acylation of fluorobenzene with benzoyl chloride was investigated over the trifluoromethanesulfonic acid (TfOH) and rare earth triflate (Re(OTf)3) composite catalyst. The results showed that the presence of Re(OTf)3 leads to the decrease in a half amount of TfOH catalyst, and there is a synergistic effect between TfOH and Re(OTf)3 in this composite catalyst. For the solvent-free acylation of fluorobenzene with benzoyl chloride, the catalytic activities of 11 kinds of rare earth elements Re(OTf)3 were tested, and it was found that among all Re(OTf)3 compounds La(OTf)3 exhibits the best catalytic performance and it can be repeatedly used. After the acylation of fluorobenzene with benzoyl chloride over the La(OTf)3 and TfOH catalyst at 140 ℃ for 4 h, the selectivity and yield to para-product fluorobenzophenone reach up to 99% and 87%, respectively. For the acylation of different benzene derivatives with benzoyl chloride over the TfOH and Re(OTf)3 catalyst, the promoting catalysis of different elements Re(OTf)3 is different, for instance, for the acylation of dimethylbenzene, Ce(OTf)3 exhibits the best catalytic performance among the tested Re(OTf)3 promoters.

[1] Wen, R. Organic Reactions for Drug Synthesis, Chemical Industry Press, Beijing, 2010, pp. 87~125 (in Chinese). (闻韧, 药物合成反应, 化学工业出版社, 北京, 2010, pp. 87~125.)
[2] Cai, H.-T.; Li, D.-D.; Liu, Z.; Wang, G.-W. Acta Chim. Sinica 2013, 71, 717 (in Chinese). (蔡海婷, 李丹丹, 刘姿, 王官武, 化学学报, 2013, 71, 717.)
[3] Corma, A.; García, H. Chem. Rev. 2003, 103, 4307.
[4] Huang, Z.-L.; Jin, L.-Q.; Lei, A.-W. Chin. J. Org. Chem. 2011, 31, 775 (in Chinese). (黄志良, 靳立群, 雷爱文, 有机化学, 2011, 31, 775.)
[5] Qiao, X.-L.; Hu, X.-Y.; Fang, Y. Chem. Ind. Eng. Prog. 2012, 12, 2702 (in Chinese). (乔兴龙, 胡学一, 方云, 化工进展, 2012, 12, 2702.)
[6] Pei, W.; Li, F.-J.; Wang, H.-B.; Sun, L.; Li, X.-N. Chin. J. Org. Chem. 2011, 31, 1188 (in Chinese). (裴文, 李风军, 王海滨, 胡香凝, 孙莉, 李小年, 有机化学, 2011, 31, 1188.)
[7] Sartori, G.; Maggi, R. Chem. Rev. 2011, 111, 181.
[8] Desmurs, J. R.; Labrouilère, M.; Le, R. C.; Gaspard, H.; Laporterie, A.; Dubac, J. Tetrahedron Lett. 1997, 38, 8871.
[9] Olah, G. A.; Farooq, O.; Morteza, S.; Farnia, F.; Olah, J. A. J. Am. Chem. Soc. 1988, 110, 2565.
[10] Procopio, A.; Dalpozzo, R.; Nino, A. D.; Maiuolo, L.; Russo, B.; Sindonab, G. Adv. Synth. Catal. 2004, 346, 1465.
[11] Fillion, E.; Fishlock, D. Tetrahedron 2009, 65, 6682.
[12] Shen, W.-P.; Zhang, J.-Y.; Zhou, H.; Qu, N. CN 101462931, 2009 [Chem. Abstr. 2009, 151, 173060].
[13] Kawada, A.; Mitamura, S.; Kobayashi, S. J. Chem. Soc., Chem. Commun. 1993, 14, 1157.
[14] Kobayashi, S.; Lwamoto, S. Tetrahedron Lett. 1998, 39, 4697.
[15] Yi, W.-B.; Cai, C. J. Fluorine Chem. 2005, 126, 1191.
[16] Dzudza, A.; Marks, T. J. J. Org. Chem. 2008, 73, 4004.
[17] Hanamoto, T.; Sugimoto, Y.; Jin, Y. Z.; Inanaga, J. Bull. Chem. Soc. Jpn. 1997, 70, 1421.
/
| 〈 |
|
〉 |