

甘二肽甲酯修饰的新型4-酰基吡唑啉酮衍生物的合成、结构和生物活性
收稿日期: 2013-06-17
修回日期: 2013-07-17
网络出版日期: 2013-08-12
基金资助
国家星火计划(No. 2010GA610009)和天津农学院科技发展基金(No. 2011N06)资助项目.
Synthesis, Structure and Biological Activities of New 1-Phenyl-3-methyl-4-acyl-2-pyrzolin-5-one Derivatives Modified with Glycylglycine Methyl Ester
Received date: 2013-06-17
Revised date: 2013-07-17
Online published: 2013-08-12
Supported by
Project supported by the China Spark Program (No. 2010GA610009) and the Science Development Fund of Tianjin Agricultural College (No. 2011N06).
朱华玲 , 班立桐 , 石军 , 陈晨 , 尉震 . 甘二肽甲酯修饰的新型4-酰基吡唑啉酮衍生物的合成、结构和生物活性[J]. 有机化学, 2013 , 33(12) : 2631 -2636 . DOI: 10.6023/cjoc201306021
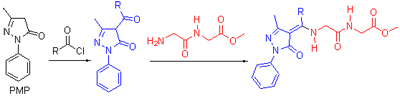
Four new 1-phenyl-3-methyl-4-acyl-2-pyrazolin-5-one derivatives modified with glycylglycine methyl ester were synthesized and characterized by UV, 1H NMR, 13C NMR, IR, elemental analysis. The molecular structure of compound b3 was also characterized by single-crystal X-ray diffraction. The analytical results showed that new compounds existed in enamine-ketone forms. The antibacterial activity tests of the compounds at different concentrations against E. cocli and Bacillus subtilis were performed using disc diffusion method. The results indicated that most compounds had weak abilities of inhibiting the growth of the two bacteria, and the inhibiting abilities of 4-aryl compounds were better. The herbicidal activity tests of the compounds against wheat and rape were performed by plate culture method. The results indicated that all compounds had the abilities of inhibiting the growth of the two plants, and the inhibiting abilities of the compounds against rape were better, especially the inhibiting abilities to the growth of rapeseed radicle almost reached completely suppressed.
Key words: pyrazolotone; glycylglycine ester; structure; biological activity
[1] Rosu, T.; Pahontu, E.; Maxim, C.; Georgescu, R.; Stanica, N.; Almajan, G. L.; Gulea, A. Polyhedron 2010, 29(2), 757.
[2] Jayarajan, R.; Vasuki, G.; Rao, P. S. Org. Chem. Int. 2010, 7, 589.
[3] Zhang, S. M.; Jia, Y. J.; Wang, J. L. J. Tianjin Normal Univ. (Nat. Sci.) 2003, 23(2), 4 (in Chinese). (张姝明, 贾永金, 王瑾玲, 缪方明, 天津师范大学学报(自然科学版), 2003, 23(2), 4.)
[4] Li, J. Z.; Jiang, L.; An, Y. M. Chin. J. Rare Earth 2004, 22(2), 189 (in Chinese). (李锦州, 蒋礼, 安郁美, 中国稀土学报, 2004, 22(2), 189.)
[5] Yu, Z. G.; Ma, J.; Cui, D. S.; Zhang, L. N. Chem. Reag. 2009, 31(4), 241 (in Chinese). (俞志刚, 马冀, 崔德生, 张丽娜, 化学试剂, 2009, 31(4), 241.)
[6] Ouyang, G. P.; Zhang, P. Q.; Xu, G. F.; Song, B. A.; Yang, S.; Jin, L. H.; Xue, W.; Hu, D. Y.; Lu, P.; Chen, Z. Molecules 2006, 11, 383.
[7] Farghaly, A. M.; Chaaban, I.; El. K., El S. M.; Fahmy S. M. J. Pharm. Sci. 1989, 3(2), 158.
[8] Yang, Z. Y.; Wang, B. D.; Li, Y. H. J. Organomet. Chem. 2006, 691, 4159.
[9] Yang, Z. Y.; Yang, R. D.; Li, R. S.; Yu, K. B. Polyhedron 2000, 19, 2599.
[10] Li, J.; Zhang, L.; Liu, L.; Liu, G. F.; Guo, J. X.; Jia, D. Z. Inorg. Chim. Acta 2007, 360, 3504.
[11] Wang, Y.; Yang, Z. Y. J. Lumin. 2008, 128, 373.
[12] Li, H. G.; Yang, Z. Y.; Qin, D. D. Inorg. Chem. Commun. 2009, 12, 494.
[13] El-Hendawy, A. M.; Al-Kubaisi, A. H.; Shoair, A. F. Monatsh. Chem. 1995, 126(12), 1291.
[14] Xu, G. C.; Zhang, L.; Liu, L.; Liu, G. F.; Jia, D. Z. Polyhedron 2008, 27, 12.
[15] Li, H. G.; Yang, Z. Y.; Wang, B. D.; Wu, J. C. J. Organomet. Chem. 2010, 695, 415.
[16] Liu, L.; Jia, D. Z.; Yu, K. B. Acta Chim. Sinica 2002, 60(3), 493 (in Chinese). (刘浪, 贾殿赠, 郁开北, 化学学报, 2002, 60(3), 493.)
[17] Guo, M.-X. M.S. Thesis, Xinjiang University, Wulumuqi, 2012 (in Chinese). (郭明晰, 硕士论文, 新疆大学, 乌鲁木齐, 2012.)
[18] Chai, H.; Liu, L.; Liu, G. F.; Jia, D. Z. Chin. J. Org. Chem. 2004, 24, 327 (in Chinese). (柴卉, 刘浪, 刘广飞, 贾殿赠, 有机化学, 2004, 24, 327.)
[19] Agar, W. T.; Hird, F. J. R.; Sidhu, G. S. J. Phys. 1953, 121(2), 255.
[20] Le, G. W.; Shi, Y. H. Chin. J. Anim. Vet. Sci. 1997, 28(6), 481 (in Chinese). (乐国伟, 施用晖, 畜牧兽医学报, 1997, 28(6), 481.)
[21] Zhang, Y. M.; Wang, X. C. J. Xibei Normal Univ. (Nat. Sci.) 1998, 34(4), 105 (in Chinese). (张有明, 王秀春, 西北师范大学学报(自然科学版), 1998, 34(4), 105.)
[22] Feng, X., Liang, S. L., Li, X. F. J. Chem. Ind. Eng. 2003, 54(2), 209 (in Chinese). (冯霞, 梁世乐, 李晓锋, 化工学报, 2003, 54(2), 209.)
[23] Lu, Z. P.; Wu, Z. S.; Yan, Z. H. J. Huazhong Normal Univ. (Nat. Sci.) 1992, 2, 013 (in Chinese). (卢治平, 吴自慎, 严振寰, 华中师范大学学报(自然科学版), 1992, 2, 013.)
[24] Tan, C. Y.; Zhang, X.; Jiang, Y. F.; Chen, J. H. Chin. Pharmacol. Bull. 2007, 23(2), 233 (in Chinese). (谭婵媛, 张昕, 姜银凤, 张冬梅, 陈钧辉, 中国药理学通报, 2007, 23(2), 233.)
[25] Wang, X. Y.; Fan, H.; Li, D. J. Prev. Med. Inf. 2009, 25(9), 730 (in Chinese). (王兴涌, 范华, 李栋, 预防医学情报杂志, 2009, 25(9), 730.)
[26] Zhang, Y. W.; Wang, Q. China Oils Fats 2007, 32(2), 69 (in Chinese). (张宇吴, 王强, 中国油脂, 2007, 32(2), 69.)
[27] Baruah, B.; Das, S.; Chakravorty, A. Inorg. Chem. 2002, 41, 4502.
[28] Garcia-Raso, A.; Fiol, J. J.; Badenas, F.; Lago, E.; Molins, E. Polyhedron 2001, 20, 2877.
[29] Yang, C. T.; Moubaraki, B.; Murray, K. S.; Vittal, J. J. J. Chem. Soc., Dalton Trans. 2003, 880
[30] Du, H.; Li, J.-Z.; Li, L. J. Mol. Sci. 2006, 22(2), 105 (in Chinese). (杜华, 李锦州, 李玲, 分子科学学报, 2006, 22(2), 105.)
[31] Li, A.-X.; Yang, Y.; Wang, J. L. Chin. J. Appl. Chem. 2004, 21(1), 49 (in Chinese). (李爱秀, 杨云, 王瑾玲, 应用化学, 2004, 21(1), 49.)
[32] Zhu, H. L.; Zhu, J. H.; Bu, L. X.; Shi, J.; Wang, J. Acta Crystallogr. 2012, E68, o1368.
[33] Li, X. Y.; Wu, Y. Q.; Gu, D. H.; Gan, F. X. Appl. Phys. A 2011, 102, 719.
[34] Zhang, X.; Zhu, H. L.; Xv, H. Z.; Wang. J. L. Chin. J. Org. Chem. 2004, 24(2), 195 (in Chinese). (张欣, 朱华玲. 徐海珍, 王瑾玲, 有机化学, 2004, 24(2), 195.)
[35] Yu, W. J.; Li, J. Z.; Li, G. Chin. J. Inorg. Chem. 1999, 15(5), 657 (in Chinese). (于文锦, 李锦州, 李刚, 无机化学学报, 1999, 15(5), 657.)
[36] Pan, L.; Chen, Y. W.; Liu, Z.; Li, Z. M. Chin. J. Org. Chem. 2013, 33, 542 (in Chinese). (潘里, 陈有为, 刘卓, 李永红, 李正名, 有机化学, 2013, 33, 542.)
[37] Yao, M. X.; An, Y.; Yan, J.; Tian, X.; Wei. S. Chin. J. Org. Chem. 2013, 33, 1015 (in Chinese). (姚明星, 安悦, 闫杰, 田星, 魏诗, 有机化学, 2013, 33, 1015.)
/
| 〈 |
|
〉 |