

手性氨基酸衍生物的合成:硅胶促进的取代氮丙啶的开环反应
收稿日期: 2013-07-05
修回日期: 2013-08-10
网络出版日期: 2013-08-16
基金资助
河北省自然科学基金(No. 2011202087)资助项目.
Synthesis of Chiral Amino-acids Derivatives:Silica-gel Promoted Ring Opening of Substituted Aziridines
Received date: 2013-07-05
Revised date: 2013-08-10
Online published: 2013-08-16
Supported by
Project supported by the Natural Science Foundation of Hebei Province (No. 2011202087).
李小娜 , 周宏勇 , 张鹏亮 , 王家喜 . 手性氨基酸衍生物的合成:硅胶促进的取代氮丙啶的开环反应[J]. 有机化学, 2013 , 33(12) : 2545 -2550 . DOI: 10.6023/cjoc201307007
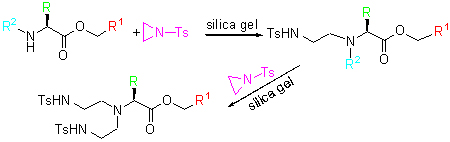
Chiral amine is a kind of important chemicals. Several L-amino acid based chiral diamines and triamines were prepared by ring-opening of substituted aziridines with L-amino acid esters in the matrix of silica without solvent. The ring-opening reactions were promoted by silica and accelerated by ultrasound radiation. The L-amino acid esters reacted with substituted aziridine 2 to give diamine 3 at first, then diamine 3 reacted with aziridine 2 again to form triamine 4. Compared with L-amino acid esters 1, compound 3 had a higher activity. (S)-Methyl pyrrolidine-2-carboxylate (1d) reacted with (R)-2-isopropyl-1-tosylaziridine (5) selectively yielding (S)-methyl 1-[(R)-3-methyl-2-(4-methylphenyl sulfonamide)butyl]pyr-rolidine-2-carboxylate (6b) through the ring opening at 1,3-position of aziridine. All obtained compounds were characterized by 1H NMR, 13C NMR, IR and elemental analysis. 4c was analyzed by X-ray diffraction as well.
[1] Chandrasekhar, S.; Narsihmulu, C.; Shameem Sultana, S. Tetrahedron Lett. 2002, 43, 7361 and references cited therein.
[2] (a) Ikariya, T.; Blacker, A. J. Acc. Chem. Res. 2007, 40, 1300.
(b) Palmer, M. J.; Wills, M. Tetrahedron: Asymmetry 1999, 10, 2045.
(c) Zhang, B.; Wang, H.; Lin, G. Q.; Xu, M. H. Eur. J. Org. Chem. 2011, 4205.
(d) Jiang, Y. T.; Jiang, Q. Z.; Zhang, X. M. J. Am. Chem. Soc. 1998, 120, 3817.
(e) Geoghegan, P.; O'Leary, P. Tetrahedron: Asymmetry 2010, 21, 867.
[3] Scheuermann, J. E. W.; Ilyashenko, G.; Griffiths, D. V.; Watkinson, M. Tetrahedron: Asymmetry 2002, 13, 269.
[4] (a) Bisai, A.; Prasad, B. A. B.; Singh, V. K. Tetrahedron Lett. 2005, 46, 7935.
(b) Thierry, J.; ServaJean, V. Tetrahedron Lett. 2004, 45, 821.
(c) Parrodi, C. A.; Vázquez, V.; Quintero, L.; Juaristi, E. Synth. Commun. 2001, 31, 3295.
(d) Yadav, J. S.; Reddy, B. V. S.; Jyothirmai, B.; Murty, M. S. R. Synlett 2002, 53.
[5] Sekar, G.; Singh, V. K. J. Org. Chem. 1999, 64, 2537.
[6] Rinaudo, G.; Narizuka, S.; Askari, N.; Crousse, B.; Bonnet-Delpon, D. Tetrahedron Lett. 2006, 47, 2065.
[7] Ye, W.; Leow, D.; Goh, S. L. M.; Tan, C. T.; Chian, C. H.; Tan, C. H. Tetrahedron Lett. 2006, 47, 1007.
[8] (a) Mori, A.; Abet, H.; Inoue, S. Appl. Organomet. Chem. 1995, 9, 189.
(b) Paradowska, J.; Stodulski, M.; Mlynarski, J. Angew. Chem., Int. Ed. 2009, 48, 4288.
[9] Urbanczyk-Lipkowska, B. Z.; KraJewski, J. W.; Gluzinski, P. Acta Crystallogr. 1982, B38, 971.
[10] Yagi, Y.; Tanaka, I.; Yamane, T.; Ashida, T. J. Am. Chem. Soc. 1983, 105, 1242.
[11] Martin, A. E.; Ford, T. M.; Bulkowski, J. E. J. Org. Chem. 1982, 47, 412.
[12] Sheldrick, G. M. SHELXS-97, University of Göttingen, Göttingen, Germany, 1997.
/
| 〈 |
|
〉 |