

新型1H-吡唑-4-甲酰胺类衍生物的合成及杀螨活性
收稿日期: 2013-07-04
修回日期: 2013-08-01
网络出版日期: 2013-08-29
基金资助
“十二五”国家科技攻关计划项目(No. 2011BAE06B03-01)和浙江省重点科技创新团队(No. 2010R50018)资助项目.
Synthesis and Acaricidal Activity of New 1H-Pyrazole-4-carboxamide Derivatives
Received date: 2013-07-04
Revised date: 2013-08-01
Online published: 2013-08-29
Supported by
Project supported by the National Key Technologies R&D Program (No. 2011BAE06B03-01) and the Key Innovation Team of Science and Technology in Zhejiang Province (No. 2010R50018).
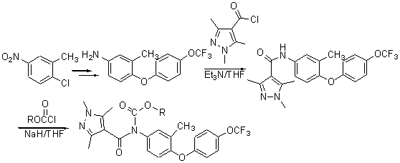
为寻求具有较高生物活性的农药新品种,采用活性基团拼接法,将1,3,5-三甲基-1H-吡唑-4-甲酰胺结构和二芳基醚结构进行活性亚结构拼接,设计并合成了10个未见文献报道的N-烷氧羰基-N-芳基取代的1H-吡唑-4-甲酰胺类化合物,其结构均经1H NMR,MS和元素分析确证,同时对所有化合物进行了初步杀螨活性测试. 活性测试结果表明,在500 mg/L质量浓度下,目标化合物对朱砂叶螨均有较强的抑制作用,且大部分化合物对朱砂叶螨的致死率达90.0%以上.
关键词: 1H-吡唑-4-甲酰胺; 合成; 杀螨活性
谢峰 , 刘婷婷 , 杨果 , 袁静 , 孔小林 , 许天明 , 谭成侠 . 新型1H-吡唑-4-甲酰胺类衍生物的合成及杀螨活性[J]. 有机化学, 2013 , 33(12) : 2596 -2601 . DOI: 10.6023/cjoc201307006
To find new lead compounds with high acaricidal bioactivity, ten new 1H-pyrazole-4-carboxamide derivatives were designed and synthesized by combining with the 1,3,5-trimethyl-1H-pyrazole-4-carboxamide moiety in pyflubumide and diaryl ether moiety in flufenoxuron. Their structures were confirmed by 1H NMR, MS and elemental analyses. The acaricidal activities of these compounds were evaluated by pesticide bioassay procedure. The results showed that most of title compounds exhibited significant effect against Tetranychus cinnabarinus with 90.0% death rate at 500 mg/L.

Key words: 1H-pyrazole-4-carboxamide; synthesis; acaricidal activity
[1] Weng, J. Q.; Wang, L.; Liu, X. H. J. Chem. Soc. Pak. 2012, 34, 1248.
[2] Liu, X. F.; Liu, X. H. Acta Crystallogr. 2011, E67, O202.
[3] Liu, X. H.; Zhao, W. G.; Wang, B. L.; Li, Z. M. Res. Chem. Intermed. 2012, 38(8), 1999.
[4] Tong, J. Y.; Shi, Y. X.; Liu, X. H.; Sun, N. B.; Li, B. J. Chin. J. Org. Chem. 2012, 32, 2373 (in Chinese). (童建颖, 石延霞, 刘幸海, 孙娜波, 李宝聚, 有机化学, 2012, 32, 2373.)
[5] Lu, L. G.; Wang, X.; Yang, S. S.; Dong, X. L.; Tang, K.; Yu, H. J. Acta Chim. Sinica 2012, 70, 192 (in Chinese). (卢林刚, 王晓, 杨守生, 董希琳, 唐凯, 余厚珺, 化学学报, 2012, 70, 192.)
[6] Yan, T.; Yu, S. J.; Liu, P. F.; Liu, Z.; Wang, B. L.; Xiong, L. X.; Li, Z. M. Chin. J. Chem. 2012, 30, 919.
[7] Yang, X. H.; Zhang, P. H.; Zhou, Y. H.; Wang, J. S.; Liu, H. J. Chin. J. Chem. 2012, 30, 672.
[8] Zhang, X. Y.; Li, Y. S.; Weng, J. Q.; Tan, C. X. Chin. J. Org. Chem. 2011, 31, 1295 (in Chinese). (张向阳, 李永曙, 翁建全, 谭成侠, 有机化学, 2011, 31, 1295).
[9] Liu, X. H.; Tan, C. X.; Weng, J. Q. Phosphorus, Sulfur Silicon Relat. Elem. 2011, 186(3), 558.
[10] Takashi, F.; Hideo, K.; Kozo, M.; Akiyuki, S.; Noriaki, Y.; Shinsuke, F. US 20090105325, 2009 [Chem. Abstr. 2009, 146, 274360].
[11] Wang, M.-H.; Yang, C.-L.; Jiang, M.-G. Word Pestic. 2002, 24(2), 13 (in Chinese). (王鸣华, 杨春龙, 蒋木庚, 世界农药, 2002, 24(2), 13.)
[12] Gon, G.-J.; Luo, G.-H. Pesticide 1990, 29(6), 4 (in Chinese). (龚国玑, 罗光宏, 农药, 1990, 29(6), 4.)
[13] Hu, W.-Q.; Zhu, W.-G.; Chen, D.-H. Chin. J. Pestic. Sci. 2007, 9(3), 240 (in Chinese). (胡伟群, 朱卫刚, 陈定花, 农药学学报, 2007, 9(3), 240.)
[14] Luo, Y. P.; Yang, G. F. Bioorg. Med. Chem. 2007, 15, 1716.
[15] Texier-Boullet, F., Klein, B.; Hamllin, J. Synthesis 1986, 409.
[16] Chiriac, C. I. Synthesis 1986, 753.
[17] Hotta, H.; Kato, Y.; Kurihara, H.; Minowa, N.; Morikawa, A.; Nakanishi, N.; Shimano, S.; Yamamoto, K.; Kato, Y.; Kato, Y., Shimano, S.; Morikawa, A.; Hotta, H.; Yamamoto, K.; Nakanishi, N. M. N.; Nakanishi, N.; Minowa, N.; Kurihara, H.; Kazumi, Y.; Hiroshi, K. EP 2305649, 2011 [Chem. Abstr.2011, 152, 168815].
[18] Prime, M. E.; Andersen, O. A.; Barker, J. J.; Brooks, M. A.; Cheng, R. K. Y.; Toogood-Johnson, I.; Courtney, S. M.; Brookfield, F. A.; Yarnold, C. J.; Marston, R. W.; Johnson, P. D.; Johnsen, S. F.; Palfrey, J. J.; Vaidya, D.; Erfan, S.; Ichihara, O.; Felicetti, B.; Palan, S.; Pedret-Dunn, A.; Schaertl, S.; Sternberger, I.; Ebneth, A.; Scheel, A.; Winkler, D.; Toledo-Sherman, L.; Beconi, M.; Macdonald, D.; Munoz-Sanjuan, I.; Dominguez, C.; Wityak, J. J. Med. Chem. 2012, 55, 1021.
[19] (a) Xue, Y. L.; Liu, X. H.; Zhang, Y. G. Asian J. Chem. 2012, 24, 1571.
(b) Xue, Y. L.; Zhang, Y. G.; Liu, X. H. Asian J. Chem. 2012, 24, 3016.
(c) Xue, Y. L.; Zhang, Y. G.; Liu, X. H. Asian J. Chem. 2012, 24, 5087.
(d) Wu, R.; Zhu, C.; Du, X. J.; Xiong, L. X.; Yu, S. J.; Liu, X. H.; Li, Z. M.; Zhao, W. G. Chem. Cent. J. 2012, 6, 99.
[20] Hideaki, M..; Koichi, R. US 20100286427, 2010 [Chem. Abstr. 2010, 150, 514885].
/
| 〈 |
|
〉 |