

过渡金属催化烷基羧酸的脱羧偶联反应研究进展
收稿日期: 2013-07-09
修回日期: 2013-08-25
网络出版日期: 2013-08-29
基金资助
国家自然科学基金(Nos. 21272050,21072040)资助项目.
Recent Advances in Transition Metal Catalyzed Decarboxylative Coupling Reactions of Alkyl Carboxylic Acid
Received date: 2013-07-09
Revised date: 2013-08-25
Online published: 2013-08-29
Supported by
Project Supported by the National Natural Science Foundation of China (Nos. 21272050, 21072040).
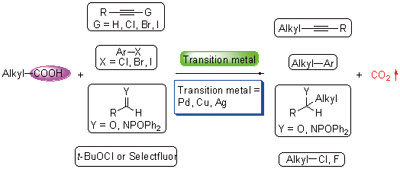
羧酸是一类廉价、易得、容易存储运输的化合物. 自芳基羧酸的脱羧Heck反应被报道以来,发展过渡金属催化的脱羧偶联反应引起了人们的广泛兴趣并已成为当前的一个研究热点. 过渡金属催化的烷基偶联反应对合成非平面的有机分子具有重要意义. 因此,发展过渡金属催化的烷基羧酸脱羧偶联反应得到了人们的广泛重视. 我们从催化脱羧偶联的过渡金属出发,分别总结了:(1)铜催化脯氨酸衍生物与炔烃(Csp3—Csp)、吲哚(Csp3—Csp2)、硝基甲烷(Csp3—Csp2)等亲核试剂的脱羧偶联反应、丙二酸单硫酯和醛的脱羧Aldol加成反应、Mannich型脱羧加成反应以及和炔溴的脱羧偶联反应;(2)钯催化吡啶-2-乙酸盐、2,2-二取代氰基乙酸、硝基取代苯乙酸等底物和卤代芳烃的脱羧偶联反应;(3)银催化烷基羧酸的脱羧氯化、氟化以及炔化反应.
戴建军 , 王光祖 , 徐小岚 , 许华建 . 过渡金属催化烷基羧酸的脱羧偶联反应研究进展[J]. 有机化学, 2013 , 33(12) : 2460 -2468 . DOI: 10.6023/cjoc201307014
Carboxylic acids are cheap, readily available, easy to store and handle compounds. Since the studies of decarboxylative Heck reaction of aryl carboxylic acids, transition metal-catalyzed decarboxylative coupling reactions have become an interesting research area. Transition metal-catalyzed alkyl coupling plays an important role in the synthesis of nonplanar molecule. Thus, much attention has been paid to the development of transition metal-catalyzed decarboxylative cross-coupling reactions of alkyl carboxylic acids. This review focuses on: (1) the copper-catalyzed decarboxylative coupling reaction between proline derivatives and nucleophilies including terminal alkynes (Csp3—Csp), indoles (Csp3—Csp2) and nitromethane (Csp3—Csp2), the copper-catalyzed decarboxylative aldol reaction of thioester derivatives, the copper-catalyzed decarboxylative Mannich-type reaction; the copper-catalyzed decarboxylative alkynylation of quaternary α-cyano acetate salts; (2) the palladium-caytalyzed decarboxylative couplings of 2-(2-azaaryl)acetates, cyanoacetate, salts, nitrophenyl acetates salts with aryl halides; (3) the silver-catalyzed decarboxylative chlorination, fluorination and alkynylation.

[1] (a) Johansson Seechurn, C. C. C.; Kitching, M. O.; Colacot, T. J.; Snieckus, V. Angew. Chem., Int. Ed. 2012, 51, 5062.
(b) Cheng, Y.; Sun, L. Chin. J. Org. Chem. 2013, 33, 877 (in Chinese). (成宜娟, 孙丽萍, 有机化学, 2013, 33, 877.)
(c) Yang, J.; Deng, M.; Yu, T. Chin. J. Org. Chem. 2013, 33, 693 (in Chinese). (杨军, 邓敏智, 于涛, 有机化学, 2013, 33, 693.)
(d) Deng, W.; Liu, L.; Guo, Q.-X. Chin. J. Org. Chem. 2004, 24, 150 (in Chinese). (邓维, 刘磊, 郭庆祥, 有机化学, 2004, 24, 150.)
(e) Xu, H.-J.; Man, Q.-S.; Lin, Y.-C.; Li, Y.-Y.; Feng, Y.-S. Chin. J. Org. Chem. 2010, 30, 9 (in Chinese). (许华建, 蔄秋石, 林义成, 李源源, 冯乙巳, 有机化学, 2010, 30, 9.)
(f) Qing, F. Chin. J. Org. Chem. 2012, 32, 815 (in Chinese). (卿凤玲, 有机化学, 2012, 32, 815.)
(g) Pan, F.; Shi, Z.-J. Acta Chim. Sinica 2012, 70, 1679 (in Chinese) (潘菲, 施章杰, 化学学报, 2012, 70, 1679.)
(h) Zhang, D.; Qin, Y. Acta Chim. Sinica 2013, 71, 147 (in Chinese) (张丹, 秦勇, 化学学报, 2013, 71, 147.)
(i) Wen, Y.-M.; Jiang, H.-F. Acta Chim. Sinica 2012, 70, 1716 (in Chinese). (温燕梅, 江焕峰, 化学学报, 2012, 70, 1716.)
(j) Zhang, B.-B.; Zhan, D.; Zhang, X.-P.; Xiang, Q.-J.; Zeng, Q.-L. Acta Chim. Sinica 2012, 70, 1655 (in Chinese). (张斌彬, 詹丹, 张小平, 向沁洁, 曾庆乐, 化学学报, 2012, 70, 1655.)
[2] (a) Baudoin, O. Angew. Chem., Int. Ed. 2007, 46, 1373.
(b) Goossen, L. J.; Rodriguez, N.; Goossen, K. Angew. Chem., Int. Ed. 2008, 47, 3100.
(c) Rodriguez, N.; Goossen, L. J. Chem. Soc. Rev. 2011, 40, 5030.
(d) Shang, R.; Liu, L. Sci. China Chem. 2011, 54, 1670.
[3] (a) Myers, A. G.; Tanaka, D.; Mannion, M. R. J. Am. Chem. Soc. 2002, 124, 11250.
(b) Tanaka, D.; Myers, A. G. Org. Lett. 2004, 6, 433.
(c) Tanaka, D.; Romeril, S. P.; Myers, A. G. J. Am. Chem. Soc. 2005, 127, 10323.
[4] (a) Goossen, L. J.; Deng, G.; Levy, L. M. Science 2006, 313, 662.
(b) Goossen, L. J.; Rodriguez, N.; Melzer, B.; Linder, C.; Deng, G.; Levy, L. M. J. Am. Chem. Soc. 2007, 129, 4824.
(c) Goossen, L. J.; Melzer, B. J. Org. Chem. 2007, 72, 7473.
(d) Goossen, L. J.; Zimmermann, B.; Knauber, T. Angew. Chem., Int. Ed. 2008, 47, 7103.
(e) Goossen, L. J.; Knauber, T. J. Org. Chem. 2008, 73, 8631.
(f) Goossen, L. J.; Rodriguez, N.; Linder, C. J. Am. Chem. Soc. 2008, 130, 15248.
(g) Goossen, L. J.; Manojolinho, F.; Khan, B. A.; Rodriguez, N. J. Org. Chem. 2009, 74, 2620.
(h) Goossen, L. J.; Rudolphi, F.; Oppel, C.; Rodriguez, N. Angew. Chem., Int. Ed. 2008, 47, 3043.
(i) Goossen, L. J.; Rodriguez, N.; Lange, P.; Linder, C. Angew. Chem., Int. Ed. 2010, 49, 1111.
(j) Goossen, L. J.; Linder, C.; Rodriguez, N.; Lange, P. P. Chem.-Eur. J. 2009, 15, 9336.
(k) Goossen, L. J.; Lange, P. P.; Rodriguez, N.; Linder, C. Chem.-Eur. J. 2010, 16, 3906.
[5] (a) Forgione, P.; Brochu, M. C.; St-Onge, M.; Thesen, K. H.; Bailey, M. D.; Bilodeau, F. J. Am. Chem. Soc. 2006, 128, 11350.
(b) Bilodeau, F.; Brochu, M. C.; Guimond, N.; Thesen, K. H.; Forgione, P. J. Org. Chem. 2010, 75, 1550.
[6] (a) Shang, R.; Fu, Y.; Wang, Y.; Xu, Q.; Yu, H.-Z.; Liu, L. Angew. Chem., Int. Ed. 2009, 48, 9350.
(b) Shang, R.; Xu, Q.; Jiang, Y.-Y.; Wang, Y.; Liu, L. Org. Lett. 2010, 12, 1000.
(c) Zhang, S.-L.; Fu, Y.; Shang, R.; Guo, Q.-X.; Liu, L. J. Am. Chem. Soc. 2010, 132, 638.
(d) Dai, J.-J.; Liu, J.-H.; Luo, D.-F.; Liu, L. Chem. Commun. 2011, 47, 677.
[7] Shang, R.; Fu, Y.; Li, J.-B.; Zhang, S.-L.; Guo, Q.-X.; Liu, L. J. Am. Chem. Soc. 2009, 131, 5738.
[8] (a) Hu, P.; Shang, Y.; Su, W. Angew. Chem., Int. Ed. 2012, 51, 5945.
(b) Hu, P.; Kan, J.; Su, W.; Hong, M. Org. Lett. 2009, 11, 2341.
(c) Fu, Z.; Huang, S.; Su, W.; Hong, M. Org. Lett. 2010, 12, 4992.
[9] Xiao, B.; Liu, Z.-J.; Liu, L.; Fu, Y. J. Am. Chem. Soc. 2013, 135, 616.
[10] (a) Lundin, P. M.; Esquivias, J.; Fu, G. C. Angew. Chem., Int. Ed. 2009, 48, 154.
(b) Strotman, N. A.; Sommer, S.; Fu, G. C. Angew. Chem., Int. Ed. 2007, 46, 3556.
(c) Zhou, J.; Fu, G. C. J. Am. Chem. Soc. 2004, 126, 1340.
(d) Lundin, P. M.; Fu, G. C. J. Am. Chem. Soc. 2010, 132, 11027.
(e) Oelke, A. J.; Sun, J.; Fu, G. C. J. Am. Chem. Soc. 2012, 134, 2966.
(f) Choi, J.; Fu, G. C. J. Am. Chem. Soc. 2012, 134, 9102.
(g) Zultanski, S. L.; Fu, G. C. J. Am. Chem. Soc. 2013, 135, 624.
(h) Lee, J.-Y.; Fu, G. C. J. Am. Chem. Soc. 2003, 125, 5616.
(i) Powell, D. A.; Maki, T.; Fu, G. C. J. Am. Chem. Soc. 2005, 127, 510.
(j) Smith, S. W.; Fu, G. C. J. Am. Chem. Soc. 2008, 130, 12645.
[11] (a) Lu, Z.; Fu, G. C. Angew. Chem., Int. Ed. 2010, 49, 6676.
(b) Smith, S. W.; Fu, G. C. Angew. Chem., Int. Ed. 2008, 47, 9334.
(c) Saito, B.; Fu, G. C. J. Am. Chem. Soc. 2007, 129, 9602.
(d) Owston, N. A.; Fu, G. C. J. Am. Chem. Soc. 2010, 132, 11908.
(e) Lu, Z.; Wilsily, A.; Fu, G. C. J. Am. Chem. Soc. 2011, 133, 8154.
(f) Wilsily, A.; Tramutola, F.; Owston, N. A.; Fu, G. C. J. Am. Chem. Soc. 2012, 134, 5794.
[12] (a) Vechorkin, O.; Hu, X. Angew. Chem., Int. Ed. 2009, 48, 2937.
(b) Vechorkin, O.; Godinat, A.; Scopelliti, R.; Hu, X. Angew. Chem., Int. Ed. 2011, 50, 11777.
(c) Vechorkin, O.; Barmaz, D.; Proust, V.; Hu, X. J. Am. Chem. Soc. 2009, 131, 12078.
(d) Csok, Z.; Vechorkin, O.; Harkins, S. B.; Scopelliti, R.; Hu, X. J. Am. Chem. Soc. 2008, 130, 8156.
(e) Vechorkin, O.; Proust, V.; Hu, X. J. Am. Chem. Soc. 2009, 131, 9756.
[13] Yang, C.-T.; Zhang, Z.-Q.; Liu, Y.-C.; Liu, L. Angew. Chem., Int. Ed. 2011, 50, 3904.
[14] Yang, C.-T.; Zhang, Z.-Q.; Liang, J.; Liu, J.-H.; Lu, X.-Y.; Chen, H.-H.; Liu, L. J. Am. Chem. Soc. 2012, 134, 11124.
[15] Yang, C.-T.; Zhang, Z.-Q.; Tajuddin, H.; Wu, C.-C.; Liang, J.; Liu, J.-H.; Fu, Y.; Czyzewska, M.; Steel, P. G.; Marder, T. B.; Liu, L. Angew. Chem., Int. Ed. 2012, 51, 528.
[16] Yi, J.; Liu, J.-H.; Liang, J.; Dai, J.-J.; Yang, C.-T.; Fu, Y.; Liu, L. Adv. Synth. Catal. 2012, 354, 1685.
[17] Bi, H.-P.; Zhao, L.; Liang, Y.-M.; Li, C.-J. Angew. Chem., Int. Ed. 2009, 48, 792.
[18] Bi, H.-P.; Teng, Q.; Guan, M.; Chen, W.-W.; Liang, Y.-M.; Yao, X.; Li, C.-J. J. Org. Chem. 2010, 75, 783.
[19] Lalic, G.; Aloise, A. D.; Shair, M. D. J. Am. Chem. Soc. 2003, 125, 2852.
[20] Magdziak, D.; Lalic, G.; Lee, H. M.; Fortner, K. C.; Aloise, A. D.; Shair, M. D. J. Am. Chem. Soc. 2005, 127, 7284.
[21] (a) Yin, L.; Kanai, M.; Shibasaki, M. J. Am. Chem. Soc. 2009, 131, 961.
(b) Yin, L.; Kanai, M.; Shibasaki, M. Tetrahedron 2012, 68, 3497.
[22] Feng, Y.-S.; Xu, Z.-Q.; Mao, L.; Zhang, F.-F.; Xu, H.-J. Org. Lett. 2013, 15, 1472.
[23] Shang, R.; Yang, Z. W.; Wang, Y.; Zhang, S.-L.; Liu, L. J. Am. Chem. Soc. 2010, 132, 14391.
[24] Jiang, Y. Y.; Fu, Y.; Liu, L. Sci. China Chem. 2012, 55, 2057.
[25] Shang, R.; Ji, D.-S.; Chu, L.; Fu, Y.; Liu, L. Angew. Chem., Int. Ed. 2011, 50, 4470.
[26] Yeung, P. Y.; Chung, K. H.; Kwong, F. Y. Org. Lett. 2011, 13, 2912.
[27] Shang, R.; Huang, Z.; Chu, L.; Fu, Y.; Liu, L. Org. Lett. 2011, 13, 4240.
[28] Xu, Z.; Wang, Q.; Zhu, J. Angew. Chem., Int. Ed. 2013, 52, 3272.
[29] Chou, C.-M.; Chatterjee, I.; Studer, A. Angew. Chem., Int. Ed. 2011, 50, 8614.
[30] Feng, Y.-S.; Wu, W.; Xu, Z.-Q.; Li, Y.; Li, M.; Xu, H.-J. Tetrahedron 2012, 68, 2113.
[31] Hyodo, K.; Kondo, M.; Funahashi, Y.; Nakamura, S. Chem. Eur. J. 2013, 19, 4128.
[32] Shang, R.; Huang, Z.; Xiao, X.; Lu, X.; Fu, Y.; Liu, L. Adv. Synth. Catal. 2012, 354, 2465.
[33] (a) Minisci, F.; Bernardi, R.; Bertini, F.; Galli, R.; Perchinummo, M. Tetrahedron 1971, 27, 3575.
(b) Minisci, F. Acc. Chem. Res. 1983, 16, 27.
[34] Cowden, C. J. Org. Lett. 2003, 5, 4497.
[35] Johnson, R. G.; Ingham, R. K. Chem. Rev. 1956, 56, 219.
[36] Wang, Z.; Zhu, L.; Yin, F.; Su, Z.; Li, Z.; Li, C. J. Am. Chem. Soc. 2012, 134, 4258.
[37] Yin, F.; Wang, Z.; Li, Z.; Li, C. J. Am. Chem. Soc. 2012, 134, 10401.
[38] Liu, X.; Wang, Z.; Cheng, X.; Li, C. J. Am. Chem. Soc. 2012, 134, 14330.
[39] Mizuta, S.; Stenhagen, I. S. R.; O'Duill, M.; Wolstenhulme, J.; Kirjavainen, A. K.; Forsback, S. J.; Tredwell, M.; Sandford, G.; Moore, P. R.; Huiban, M.; Luthra, S. K.; Passchier, J.; Solin, O.; Gouverneur, V. Org. Lett. 2013, 15, 2648.
[40] Liu, L.; Zhou, W. J.; Chruma, J. J.; Breslow, R. J. Am. Chem. Soc. 2004, 126, 8136.
[41] Liu, L.; Breslow, R. Bioorg. Med. Chem. 2004, 12, 3277.
[42] Chruma, J. J.; Liu, L.; Zhou, W. J.; Breslow, R. Bioorg. Med. Chem. 2005, 13, 5873.
/
| 〈 |
|
〉 |