

无配体Cu2O纳米线催化C—N/C—O偶联反应
收稿日期: 2013-06-14
修回日期: 2013-08-30
网络出版日期: 2013-09-17
基金资助
湖南省自然科学基金(No. 13JJ5018);湖南省科技厅支撑(No. 湘财企指(2009)76号)与长沙市科技局(No. 长财企指(No. 2012)64号)资助项目.
Ligand-Free Cu2O Nanowire Catalyzing C—N and C—O Coupling Reaction
Received date: 2013-06-14
Revised date: 2013-08-30
Online published: 2013-09-17
Supported by
Project supported by the Hunan Provincial Natural Science Foundation of China (No. 13JJ5018), the Key Technology R&D Program of Hnnan Provincial Science & Technology Department (No. 2009-76), the Changsha Science and Technology (No. 2012-64).
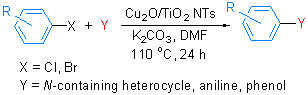
介绍了由TiO2纳米阵列管上负载Cu2O纳米线,制备新型纳米催化剂Cu2O/TiO2 NTs的方法. 应用该新型催化剂,研究了其对卤代芳烃的C—N,C—O偶联反应的催化作用,结果表明Cu2O/TiO2 NTs不仅能很好地催化溴代芳烃与氮/氧亲核试剂的反应,而且可顺利催化氯代芳烃的反应,得到中等及优良的产率. 催化剂循环使用试验证实,催化剂在该体系中能被回收利用5次收率基本稳定.
关键词: Cu2O/TiO2催化; N-芳基化; O-芳基化; 回收利用
董奇志 , 向建南 , 郭灿城 . 无配体Cu2O纳米线催化C—N/C—O偶联反应[J]. 有机化学, 2014 , 34(1) : 147 -154 . DOI: 10.6023/cjoc201306020
A novel nano-material with Cu2O nanowires loaded on TiO2 nanotubes (Cu2O/TiO2 NTs) was prepared in this paper. The new type of catalyst was used to catalyze the C—N and C—O coupling of chlorinated aromatics. The results showed that this catalyst promised the reaction of not only aryl bromide, but also aryl chloride, and most products were synthesized in good to excellent yields. Moreover, the reused experiments confirmed that the catalyst can be recycled five times and present stable yields for reaction.

Key words: Cu2O/TiO2-catalyst; N-arylation;O-arylation; recycling
[1] Xu, H. J.; Tong, Q. S.; Lin, Y. C.; Li, Y. Y.; Feng, Y. S. Chin. J. Org. Chem. 2010, 30, 9 (in Chinese).
(许华建, 蔄秋石, 林义成, 李源源, 冯乙巳, 有机化学, 2010, 30, 9.)
[2] (a) Deng, W.; Liu. L.; Guo, Q. X. Chin. J. Org. Chem. 2004, 24, 150 (in Chinese).
(邓维, 刘磊, 郭庆祥, 有机化学, 2004, 24, 150.)
(b) Wang, Y. F.; Zeng, J. H.; Cui, X. R. Chin. J. Org. Chem. 2010, 30, 181 (in Chinese).
(王晔峰, 曾京辉, 崔晓瑞, 有机化学, 2010, 30, 181.;
c) Wu, X.; Hu, W. Chin. J. Chem. 2011, 29, 2124.
(d) Yang, X.; Xing, H.; Zhang, Y.; Lai, Y.; Zhang, Y.; Jiang, Y.; Ma, D. Chin. J. Chem. 2012, 30, 875.
(e) Qian, C.; Zong, Q.; Fang, D. Chin. J. Chem. 2012, 30, 199.
(f) Jiao, Y.; Yan, N.; Xie, J.; Ma, X.; Liu, P.; Dai, B. Chin. J. Chem. 2013, 31, 267.
[3] Strieter, E. R.; Bhayana, S. B.; Buchwald, S. L. J. Am. Chem. Soc. 2009, 131, 78.
[4] Zhang, H.; Cai, Q.; Ma, D. W. J. Org. Chem. 2005, 70, 5164.
[5] Zhang, J. X.; Yin, H. Q.; Han, S. Q. Chin. J. Org. Chem. 2012, 32, 1429 (in Chinese).
(张敬先, 殷慧清, 韩世清, 有机化学, 2012, 32, 1429.)
[6] Correa, A.; Bolm, C. Adv. Synth. Cata1. 2007, 349, 2673.
[7] Manbeck, G. F.; Lipman, A. J.; Stockland, R. A.; Freidl, A. L.; Hasler, A. F.; Stone, J. J.; Guzei, I. A. J. Org. Chem. 2005, 70, 24.
[8] Jiao, J.; Zhang, X. R.; Chang, N. H.; Wang, J.; Wei, J. F. J. Org. Chem. 2011, 76, 1180.
[9] Yong, F. F.; Teo, Y. C.; Tay, S. H.; Lim, K. H. Tetrahedron Lett. 2011, 52, 1161.
[10] Sreedhar, B.; Arundhathi, R.; Reddy, P. L.; Kantam, M. L. J. Org. Chem. 2009, 74, 7951.
[11] Huang, Y. Z.; Gao, J.; Ma, H.; Miao, H.; Xu, J. Tetrahedron Lett. 2008, 49, 948.
[12] Xi, Z. X.; Liu, F. H.; Zhou, Y. B.; Chen, W. Z. Tetrahedron 2008, 64, 4254.
[13] (a) Ma, D. W.; Cai, Q. Synlett 2004, 128.
(b) Zhang, H.; Cai, Q.; Ma, D. W. J. Org. Chem. 2005, 70, 5164.
[14] (a) Maheswaran, H.; Krishna, G. G.; Prasanth, K. L. Tetrahedron 2008, 64, 2471.
(b) Xie, Y.-X.; Pi, S.-F.; Wang, J.; Yin, D. L.; Li, J. H. J. Org. Chem. 2006, 71, 8324.
[15] Anderson, K. W.; Tundel, R. E.; Ikawa, T.; Altman, R. A. Angew. Chem., Int. Ed. 2006, 45, 6523.
[16] Patil, N. M.; Kelkar, A. A.; Nabi, Z.; Chaudhari, R. V. Chem. Commun. 2003, 2460.
[17] (a) Kataoka, N.; Shelby, Q.; Stambuli, J. P.; Hartwig, J. F. J. Org. Chem. 2002, 67, 5553.
(b) Chen, H. S.; Wang, Q. R.; Tao, F. G. Chin. J. Chem. 2009, 27, 1382.
(c) Chen, G. S.; Lam, W. H.; Fok, W. S.; Lee, H. W.; Kwong, F. Y. Chem. Asian J. 2007, 2, 306.
(d) Gujadhur, R. K.; Bates, C. G.; Venkataraman, D. Org. Lett. 2001, 3, 4315.
[18] Li, F.; Wang, Q. R.; Ding, Z. B.; Tao, F. G. Org. Lett. 2003, 5, 2169.
[19] Suresh, P.; Pitchumani, K. J. Org. Chem. 2008, 73, 9121.
[20] Kantam, M. L.; Venkanna, G. T.; Sridhar, C.; Sreedhar, B.; Choudary, B. M. J. Org. Chem. 2006, 71, 9522.
[21] Ma, H.-C.; Jiang, X.-Z. J. Org. Chem. 2007, 72, 8943.
[22] Yang, M.; Liu, F. J. Org. Chem. 2007, 72, 8969.
[23] Xie, Y.-X.; Pi, S.-F.; Wang, J.; Yin, D.-L.; Li, J.-H. J. Org. Chem. 2006, 71, 8324
[24] Yang, K.; Qiu, Y.; Li, Z.; Wang, Z.; Jiang, S. J. Org. Chem. 2011, 76, 3151.
[25] Astolfi, P.; Carloni, P.; Damiani, E.; Greci, L.; Marini, M.; Rizzoli, C.; Stipa, P. Eur. J. Org. Chem. 2008, 3279.
[26] Fors, B. P.; Davis, N. R.; Buchwald, S. L. J. Am. Chem. Soc. 2009, 131, 5766
[27] Zhu, X.-H.; Chen, G.; Ma, Y.; Song, H.-C.; Xu, Z.-L.; Wan, Y.-Q. Chin. J. Chem. 2007, 25, 546.
[28] Wang, B.-A.; Zeng, R.-S.; Wei, H.-Q.; Jia, A.-Q.; Zou, J.-P. Chin. J. Chem. 2006, 24, 1062.
/
| 〈 |
|
〉 |