

焦磷酸酯合成的研究进展
Advances in the Synthesis of Organic Pyrophosphate
Received date: 2013-07-03
Revised date: 2013-08-11
Online published: 2013-09-25
侯淑华 , 曲忠国 , 汤立军 . 焦磷酸酯合成的研究进展[J]. 有机化学, 2014 , 34(1) : 54 -64 . DOI: 10.6023/cjoc201307005
The recent advances in the synthesis of organic pyrophosphate are reviewed, based on the coupling methods including dicyclohexylcarbodiimide (DCC), N,N'-carbonyldiimidazole (CDI), DCC/morpholine/MnCl2/MgSO4, DCC/ morpholine/1H-tetrazole, PPh3/(PyS)2/N-methylimidazole, diphenyl phosphorochloridate (DPCP), trifluoroacetic anhydride/N-methylimidazole. Mechanism of some reactions is also discussed.
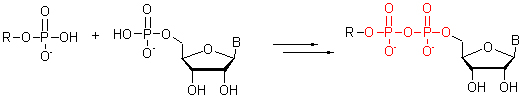
Key words: organic pyrophosphate; synthesis; advances
[1] (a) Lin, S. J.; Guarente, L. Curr. Opin. Cell Biol. 2003, 15, 241.
(b) Pollak, N.; Dolle, C.; Ziegler, M. Biochem. J. 2007, 402, 205.
(c) van der Donk, W. A.; Zhao, H. Curr. Opin. Biotechnol. 2003, 14, 421.
(d) Sauve, A. A.; Deng, H. T.; Angeletti, R. H.; Schramm, V. L. J. Am. Chem. Soc. 2000, 122, 7855.
(e) Xia, W. L.; Wang, Z.; Wang, Q.; Han, J.; Zhao, C. P.; Hong, Y. Y.; Zeng, L. L.; Tang, L.; Ying, W. H. Curr. Pharm. Des. 2009, 15, 12.
[2] (a) Leloir, L. F. Science 1971, 172, 1299.
(b) Kochetkov, N. K.; Shibaev, V. N. Adv. Carbohydr. Chem. Biochem. 1973, 28, 307.
[3] Mohnen, D. Curr. Opin. Plant Biol. 2008, 11, 266.
[4] Freeze, H. In Essentials of Glycobiology, Eds.: Varki, A.; Cummings, R.; Esko, J.; Freeze, H.; Hart, G.; Marth, J., Cold Spring Harbor Laboratory Press, New York, 1999, p. 66.
[5] (a) Thibodeaux, C. J.; Melançon, C. E.; Liu, H. W. Angew. Chem., Int. Ed. 2008, 47, 9814.
(b) Bourgeaux, V.; Piller, F.; Piller, V. Bioorg. Med. Chem. Lett. 2005, 15, 5459.
[6] Wagner, G. K.; Pesnot, T.; Field, R. A. Nat. Prod. Rep. 2009, 26, 1172.
[7] Hughes, N. H.; Kenner, G. W.; Todd, A. C. J. Chem. Soc. 1957, 3733.
[8] Dowden, J.; Moreau, C.; Brown, R. S.; Berridge, G.; Galione, A.; Potter, B. V. L. Angew. Chem., Int. Ed. 2004, 43, 4637.
[9] Hou, S. H.; Liu, W. J.; Zhao, Z. B. Chin. J. Org. Chem. 2012, 32, 349 (in Chinese).
(侯淑华, 刘武军, 赵宗保, 有机化学 2012, 32, 349.)
[10] (a) Koh, D. W.; Coyle, D. L.; Mehta, N.; Ramsinghani, S.; Kim, H.; Slama, J. T.; Jacobson, M. K. J. Med. Chem. 2003, 46, 4322.
(b) Franchetti, P.; Cappellacci, L.; Pasqualini, M.; Petrelli, R.; Jayaprakasan, V.; Jayaram, H. N.; Boyd, D. B.; Jain, M. D.; Grifantini, M. Bioorg. Med. Chem. 2005, 13, 2045.
(c) Moreau, C.; Wagner, G. K.; Weber, K.; Guse, A. H.; Potter, B. V. J. Med. Chem. 2006, 49, 5162.
(d) Das, A.; Ko, H.; Burianek, L; Barrett, M.; Harden, T.; Jacobson, K. J. Med. Chem. 2010, 53, 471.
[11] (a) Kurosu, M.; Mahapatra, S.; Narayanasamy, P.; Crick, D. C. Tetrahedron Lett. 2007, 48, 799.
(b) Takaku, H.; Sato, J.; Ishida, H.-K.; Inazu, T.; Ishida, H.; Kiso, M. Glycoconjugate J. 2006, 23, 565.
(c) Ishimizu, T.; Uchida, T.; Sano, K.; Hase, S. Tetrahedron: Asymmetry 2005, 16, 309.
(d) Pesnot, T.; Wagner, G. K. Org. Biomol. Chem. 2008, 6, 2884.
(e) Ko, H.; Fricks, I.; Ivanov, A. A.; Harden, T. K.; Jacobson, K. A. J. Med. Chem. 2007, 50, 2030.
[12] Wahler, D.; Reymond, J.-L. Can. J. Chem. 2002, 80, 665.
[13] Moffatt, J. G.; Khorana, H. G. J. Am. Chem. Soc. 1958, 80, 3756.
[14] (a) Roseman, S.; Distler, J. J.; Moffatt, J. G.; Khorana, H. G. J. Am. Chem. Soc. 1961, 83, 659.
(b) Moffatt, J. G.; Khorana, H. G. J. Am. Chem. Soc. 1961, 83, 649.
[15] Collier, A.; Wagner, G. K. Chem. Commum. 2008, 178.
[16] Lee, J.; Churchil, H.; Choi, W. B.; Lynch, J. E.; Roberts, F. E.; Volante, R. P.; Reider, P. J. Chem. Commun. 1999, 729.
[17] Kennedy, K. J.; Bressi, J. C.; Gelb, M. H. Bioorg. Med. Chem. Lett. 2001, 11, 95.
[18] Zhou, G.-C.; Parikh, S. L.; Tyler, P. C.; Evans, G. B.; Furneaux, R. H.; Zubkova, O. V.; Benjes, P. A.; Schramm, V. L. J. Am. Chem. Soc. 2004, 126, 5690.
[19] Wagner, G. K.; Guse, A. H.; Potter, B. V. J. Org. Chem. 2005, 70, 4810.
[20] Zhang, B.; Wagner, G.; Weber, K.; Garnham, C.; Morgan, A.; Galione, A.; Guse, A.; Potter, B. J. Med. Chem. 2008, 51, 1623.
[21] Wittmann, V.; Wong, C. H. J. Org. Chem 1997, 62, 2144.
[22] (a) Zhao, Z.; Liu, H.-W. J. Org. Chem. 2001, 66, 6810.
(b) Chen, H.; Zhao, Z.; Hallis, T. M.; Guo, Z.; Liu, H. W. Angew. Chem., Int. Ed. 2001, 113, 627.
(c) Hallis, T. M.; Zhao, Z.; Liu, H.-W. J. Am. Chem. Soc. 2000, 122, 10493.
[23] Zamyatina, A.; Gronow, S.; Oertelt, C.; Puchberger, M.; Brade, H.; Kosma, P. Angew. Chem., Int. Ed. 2000, 39, 4150.
[24] (a) Dinev, Z.; Wardak, A. Z.; Brownlee, R. T.; Williams, S. J. Carbohydr. Res. 2006, 341, 1743.
(b) Fairweather, J. K.; Him, J. L. K.; Heux, L.; Driguez, H.; Bulone, V. Glycobiology 2004, 14, 775.
[25] (a) Dulcey, A. E.; Qasba, P. K.; Lamb, J.; Griffiths, G. L. Tetrahedron 2011, 67, 2013.
(b) Ravalico, F.; Messina, I.; Berberian, M.; James, S.; Migaud, M.; Vyle, J. Org. Biomol. Chem. 2011, 9, 6496.
(c) Tsukamoto, H.; Kahne, D. Bioorg. Med. Chem. Lett. 2011, 21, 5050.
(d) Wu, M.; Meng, Q.; Ge, M.; Bai, L.; Zhou, H. Tetrahedron Lett. 2011, 52, 5799.
(e) Tedaldi, L.; Pierce, M.; Wagner, G. Carbohydr. Res. 2012, 364, 22.
[26] Mukaiyama, T. Phosphorus Sulfur 1976, 1, 371.
[27] Abramova, T. V.; Vasileva, S. V.; Serpokrylova, I. Y.; Kless, H.; Silnikov, V. N. Bioorg. Med. Chem. 2007, 15, 6549.
[28] (a)Hou, S.; Liu, W.; Ji, D.; Wang, Q.; Zhao, Z. K. Tetrahedron Lett. 2011, 52, 5855.
(b) Ji, D.; Wang, L.; Hou, S.; Liu, W.; Wang, J.; Wang, Q.; Zhao, Z. J. Am. Chem. Soc. 2011, 133, 20857.
(c) Ji, D.; Wang, L.; Liu, W.; Hou, S.; Zhao, Z. Sci. China Chem. 2013, 56, 296.
[29] (a) Michelson, A. Biochim. Biophys. Acta 1964, 91, 1.
(b) Ma, Q.-F.; Reynolds, M. A.; Kenyon, G. L. Bioorg. Chem. 1989, 17, 194.
[30] Mort, C. J. W.; Migaud, M. E.; Galione, A.; Potter, B. V. L. Bioorg. Med. Chem. 2004, 12, 475.
[31] Comstock, L. R.; Denu, J. M. Org. Biomol. Chem. 2007, 5, 3087.
[32] (a)Timmons, S. C.; Jakeman, D. L. Carbohydr. Res. 2008, 343, 865.
(b) Mohamady, S.; Taylor, S. J. Org. Chem., 2011, 76, 6344.
[33] Gold, H.; van Delft, P.; Meeuwenoord, N.; Codée, J. D.; Filippov, D. V.; Eggink, G.; Overkleeft, H. S.; van der Marel, G. A. J. Org. Chem. 2008, 73, 9458.
[34] (a) Wolf, S.; Berrio, R. M.; Meier, C. Eur. J. Org. Chem. 2011, 2011, 6304.
(b) Wendicke, S.; Warnecke, S.; Meier, C. Angew. Chem., Int. Ed. 2008, 47, 1500.
/
| 〈 |
|
〉 |