

镍催化酚衍生物的Suzuki-Miyaura偶联反应研究进展
收稿日期: 2013-07-23
修回日期: 2013-09-06
网络出版日期: 2013-09-25
Progress of Nickel-Catalyzed Suzuki-Miyaura Cross-Coupling Reactions of Phenol Derivatives
Received date: 2013-07-23
Revised date: 2013-09-06
Online published: 2013-09-25
过渡金属镍催化的Suzuki-Miyaura偶联反应在有机合成中有着广泛的应用. 综述了近年来以过渡金属镍催化的以酚的衍生物为底物的偶联反应的研究进展. 主要包括芳基磺酸酯、氨基磺酸芳基酯、碳酸芳基酯、芳基羧酸酯、氨基甲酸芳基酯、芳基磷酸酯、芳醚、杂芳醚和酚盐的偶联反应.
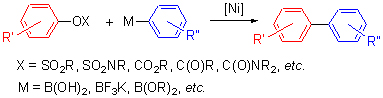
关键词: 镍; 酚衍生物; Suzuki-Miyaura偶联反应
陈国军 , 杜建时 . 镍催化酚衍生物的Suzuki-Miyaura偶联反应研究进展[J]. 有机化学, 2014 , 34(1) : 65 -80 . DOI: 10.6023/cjoc201307035
Suzuki-Miyaura coupling reaction catalyzed by transition-metal nickel is widely used in organic synthesis. In this review, the development of transition-metal nickel-catalyzed cross-coupling reactions is summarized, mainly including aryl sulfonates, aryl sulfamates, aryl carbonates, aryl carboxylates, aryl carbamates, aryl phosphorus derivatives, aryl ethers, aryl heteroaryl ethers and phenolate.
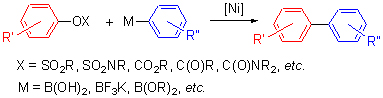
[1] Zhang, D.; Qin, Y. Acta Chim. Sinica 2013, 71, 147 (in Chinese).
(张丹, 秦勇, 化学学报, 2013, 71, 147.)
[2] Wen, Y.-M.; Jiang, H.-F. Acta Chim. Sinica 2012, 70, 1716 (in Chinese).
(温燕梅, 江焕峰, 化学学报, 2012, 70, 1716.)
[3] Pan, F.; Shi, Z.-J. Acta Chim. Sinica 2012, 70, 1679 (in Chinese).
(潘菲, 施章杰, 化学学报, 2012, 70, 1679.)
[4] Jin, L.-Q.; Luo, X.-C.; Lei, A.-W. Acta Chim. Sinica 2012, 70, 1538 (in Chinese).
(靳立群, 罗贤才, 雷爱文, 化学学报, 2012, 70, 1538.)
[5] Hassan, J.; Sevignom, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Chem. Rev. 2002, 102, 1359.
[6] Rosen, B. M.; Quasdorf, K. W.; Wilson, D. A.; Zhang, N.; Resmerita, A.-M.; Garg, N. K.; Percec, V. Chem. Rev. 2011, 111, 1346.
[7] Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457.
[8] Jana, R.; Pathak, T. P.; Sigman, M. S. Chem. Rev. 2011, 111, 1417.
[9] Han, F.-S. Chem. Soc. Rev. 2013, 42, 5270.
[10] Percec, V.; Bae, J.-Y.; Hill, D. H. J. Org. Chem. 1995, 60, 1060.
[11] Ueda, M.; Saitoh, A.; Oh-tani, S.; Miyaura, N. Tetrahedron 1998, 54, 13079.
[12] Zim, D.; Lando, V. R.; Dupont, J.; Monteiro, A. L. Org. Lett. 2001, 3, 3049.
[13] Kobayashi, Y.; Mizojiri, R. Tetrahedron Lett. 1996, 37, 8531.
[14] Kobayashi, Y.; William, A. D.; Mizojiri, R. J. Organomet. Chem. 2002, 653, 91.
[15] Tang, Z.-Y.; Hu, Q.-S. J. Am. Chem. Soc. 2004, 126 , 3058.
[16] Tang, Z.-Y.; Spinella, S.; Hu, Q.-S. Tetrahedron Lett. 2006, 47, 2427.
[17] Wilson, D. A.; Wilson, C. J.; Rosen, B. M.; Percec, V. Org. Lett. 2008, 10, 4879.
[18] Leowanawat, P.; Zhang, N.; Resmerita, A.-M.; Rosen, B. M.; Percec, V. J. Org. Chem. 2011, 76, 9946.
[19] Lipshutz, B. H.; Butler, T.; Swift, E. Org. Lett. 2008, 10, 697.
[20] Kuroda, J.; Inamoto, K.; Hiroya, K.; Doi, T. Eur. J. Org. Chem. 2009, 2251.
[21] Tu, T.; Mao, H.; Herbert, C.; Xu, M.-Z.; Dötz, K. H. Chem. Commun. 2010, 46, 7796.
[22] Molander, G. A.; Beaumard, F. Org. Lett. 2010, 12, 4022.
[23] Fan, X.-H.; Yang, L.-M. Eur. J. Org. Chem. 2010, 2457.
[24] Fan, X.-H.; Yang, L.-M. Eur. J. Org. Chem. 2011, 1467.
[25] Leowanawat, P.; Zhang, N.; Safi, M.; Hoffman, D. J.; Fryberger, M. C.; George, A.; Percec, V. J. Org. Chem. 2012, 77, 2885.
[26] Xing, C.-H.; Lee, J.-R.; Tang, Z.-Y.; Zheng, J.-R.; Hu, Q.-S. Adv. Synth. Catal. 2011, 353, 2051.
[27] Leowanawat, P.; Zhang, N.; Percec, V. J. Org. Chem. 2012, 77, 1018.
[28] Zhang, N.; Hoffman, D. J.; Gutsche, N.; Gupta, J.; Percec, V. J. Org. Chem. 2012, 77, 5956.
[29] Gao, H.; Li, Y.; Zhou, Y.-G.; Han, F.-S.; Lin, Y.-J. Adv. Synth. Catal. 2011, 353, 309.
[30] Quasdorf, K. W.; Riener, M.; Petrova, K. V.; Garg, N. K. J. Am. Chem. Soc. 2009, 131, 17748.
[31] Quasdorf, K. W.; Antoft-Finch, A.; Liu, P.; Silberstein, A. L.; Komaromi, A.; Blackburn, T.; Ramgren, S. D.; Houk, K. N.; Snieckus, V.; Garg, N. K. J. Am. Chem. Soc. 2011, 133, 6352.
[32] Baghbanzadeh, M.; Pilger, C.; Kappe, C. O. J. Org. Chem. 2011, 76, 1507.
[33] Chen, G.-J.; Han, F.-S. Eur. J. Org. Chem. 2012, 3575.
[34] Kuwano, R.; Shimizu, R. Chem. Lett. 2011, 40, 913.
[35] Guan, B.-T.; Wang, Y.; Li, B.-J.; Yu, D.-G.; Shi, Z.-J. J. Am. Chem. Soc. 2008, 130, 14468.
[36] Quasdorf, K. W.; Tian, X.; Garg, N. K. J. Am. Chem. Soc. 2008, 130, 14422.
[37] Li, Z.; Zhang, S.-L.; Fu, Y.; Guo, Q.-X.; Liu, L. J. Am. Chem. Soc. 2009, 131, 8815.
[38] Antoft-Finch, A.; Blackburn, T.; Snieckus, V. J. Am. Chem. Soc. 2009, 131, 17750.
[39] Xu, L.; Li, B.-J.; Wu, Z.-H.; Lu, X.-Y.; Guan, B.-T.; Wang, B.-Q.; Zhao, K.-Q.; Shi, Z.-J. Org. Lett. 2010, 12, 884.
[40] Zhao, Y.-L.; Li, Y.; Li, Y.; Gao, L.-X.; Han, F.-S. Chem.-Eur. J. 2010, 16, 4991.
[41] Chen, G.-J.; Huang, J.; Gao, L.-X.; Han, F.-S. Chem.-Eur. J. 2011, 17, 4038.
[42] Li, S.-M.; Huang, J.; Chen, G.-J.; Han, F.-S. Chem. Commun. 2011, 47, 12840.
[43] Chen, H.; Huang, Z.-B.; Hu, X.-M.; Tang, G.; Xu, P.-X.; Zhao, Y.-F.; Cheng, C.-H. J. Org. Chem. 2011, 76, 2338.
[44] Tobisu, M.; Shimasaki, T.; Chatani, N. Angew. Chem., Int. Ed. 2008, 47, 4866.
[45] Li, X.-J.; Zhang, J.-L.; Geng, Y.; Jin, Z. J. Org. Chem. 2013, 78, 5078.
[46] Yu, D.-G.; Shi, Z.-J. Angew. Chem., Int. Ed. 2011, 50, 7097.
/
| 〈 |
|
〉 |