

O-烷基α-(取代苯氧乙酰氧基)烃基膦酸盐的合成与生物活性
收稿日期: 2013-08-12
修回日期: 2013-09-06
网络出版日期: 2013-09-25
基金资助
国家重点基础研究发展规划(973计划,No.2010CB126100);国家自然科学基金(No. 21172090);教育部创新团队(No. IRT0953)资助项目.
Synthesis and Biological Activity of O-Alkyl α-(Substituted phenoxyacetoxy)alkylphosphonate
Received date: 2013-08-12
Revised date: 2013-09-06
Online published: 2013-09-25
Supported by
Project supported by the National Basic Research Program of China (973 Program, No. 2010CB126100), the National Natural Science Foundation of China (No. 21172090) and the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, No. IRT0953).
为了研究O-烷基α-(取代苯氧乙酰氧基)烃基膦酸衍生物的结构活性关系,以取代苯氧乙酸为起始原料,经氯化亚砜氯化后得中间体2,然后与不同取代的α-羟基烃基膦酸酯((1))反应制备关键中间体3,再与碘化钠或溴化锂反应,合成了14个未见文献报道的O-烷基α-(取代苯氧乙酰氧基)烃基膦酸盐4. 通过1H NMR,IR,MS和元素分析对所合成的化合物进行了结构表征. 初步的生物活性测试结果表明:大多数目标化合物具有较好的除草活性或杀菌活性. 化合物4a在1.5 kg/ha剂量下对所测单、双子叶植物的抑制率均达到了100%;在50 μg/g的剂量下,化合物4h~4m对油菜菌核菌和黄瓜灰霉菌的抑制率达到90%以上.
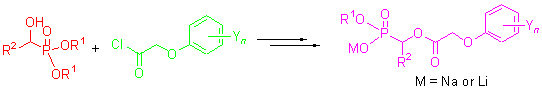
王涛 , 邹鹏 , 彭浩 , 贺红武 . O-烷基α-(取代苯氧乙酰氧基)烃基膦酸盐的合成与生物活性[J]. 有机化学, 2014 , 34(1) : 215 -219 . DOI: 10.6023/cjoc201308014
For the purpose of studying the structure-activity relationship of O-alkyl α-(substituted phenoxyacetoxy)alkylphosphonic derivatives, fourteen novel O-alkyl α-(substituted phenoxyacetoxy)alkylphosphonates were synthesized by the reactions of key intermediate 3 with NaI or LiBr. The intermediate 3 was prepared by the treatment of α-hydroxyalkylphosphonates 1 with substituted phenoxyacetyl chlorides 2 which could be easily obtained by the reaction of substituted phenoxyacetic acids with excess thionyl chloride. The structures of compounds 4 have been confirmed by 1H NMR, IR, MS and elemental analyses. The results of preliminary bioassay indicated that most of title compounds possess significant herbicidal and fungicidal activities. Compound 4a showed 100% inhibitory activity against all the tested species in the rate of 1.5 kg/ha. Compounds 4h~4m exhibited more than 90% inhibitory activity against Sclerotinia sclerotiorum and Botrytis cinerea in the rate of 50 μg/g.
Key words: synthesis; phosphonate; herbicidal activity; fungicidal activity
[1] Baillie, A. C.; Wright, K.; Wright, B. J.; Earnshaw, C. G. Pestic. Biochem. Physiol. 1988, 30, 103.
[2] Kluger, R.; Pike, D. C. J. Am. Chem. Soc. 1977, 99, 4504.
[3] Kluger, R.; Gish, G.; Kauffman, G. J. Biol. Chem. 1984, 259, 8960.
[4] He, H. W.; Yuan, J. L. Peng, H.; Chen, T.; Shen, P.; Wan, S. Q.; Li, Y. J.; Tan, H. L.; He, Y. H.; He, J. B.; Li, Y. J. Agric. Food Chem. 2011, 59, 4801.
[5] Wang, T.; He, H. W.; Miao, F. M. Chin. J. Org. Chem. 2009, 29, 1152 (in Chinese).
(贺红武, 刘建超, 缪方明, 有机化学, 2009, 29, 1152.)
[6] He, H. W.; Wang, T.; Yuan, J. L. J. Organomet. Chem. 2005, 690, 2608.
[7] Peng, H.; Wang, T.; Xie, P.; Chen, T.; He, H. W.; Wang, J. J. Agric. Food Chem. 2007, 55, 1871.
[8] Wang, T.; He, H. W. Synth. Commun. 2004, 34, 1415.
[9] He, H. W.; Peng, H.; Wang, T.; et al.Wang, C. B.; Yuan, J. L.; Chen, T.; He, J. B.; Tan, X. S., J. Agric. Food Chem. 2013, 61, 2479.
[10] Texier-Boullet, F.; Foucaud, A. Synthesis 1982, 916.
[11] Peng, H.; He, H. W. Chin. J. Org. Chem. 2007, 27, 502 (in Chinese).
(彭浩, 贺红武, 有机化学, 2007, 27, 502.)
[12] Liu, J. C.; Cui, Z. P.; He, H. W. Chin. J. Org. Chem. 2011, 31, 2082 (in Chinese).
(刘建超, 崔泽平, 贺红武, 有机化学, 2011, 31, 2082.)
[13] .Lei, J. P.; Han, J. T.; X, Z. H.; Dong, H. B.; Wang, M. A. Chin. J. Org. Chem. 2012, 32, 1993 (in Chinese).
(雷建平, 韩金涛, 徐志红, 董宏波, 王明安, 有机化学, 2012, 32, 1993.)
/
| 〈 |
|
〉 |