

香豆素并[4,3-b]吡喃-4-酮类衍生物的有效合成方法
收稿日期: 2013-07-29
修回日期: 2013-08-24
网络出版日期: 2013-09-30
Synthesis of Coumarino[4,3-b]pyran-4-one Derivatives
Received date: 2013-07-29
Revised date: 2013-08-24
Online published: 2013-09-30
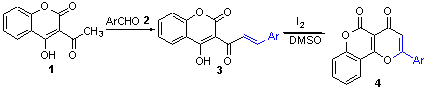
在哌啶和聚乙二醇-400的作用下,通过3-乙酰基-4-羟基香豆素与芳香醛的缩合反应,合成了3-肉桂酰基-4-羟基香豆素,接着在I2-DMSO氧化体系下,成功制备了一系列香豆素并[4,3-b]吡喃-4-酮衍生物. 该反应操作简单、条件温和、收率良好. 产物结构经NMR,IR,MS及元素分析数据得以证实.
关键词: 香豆素; 3-肉桂酰基-4-羟基香豆素, 香豆素并[4,3-b]吡喃-4-酮; 碘; 合成
王道林 , 杨菲菲 , 刘忠 , 董哲 , 赵伟 . 香豆素并[4,3-b]吡喃-4-酮类衍生物的有效合成方法[J]. 有机化学, 2014 , 34(1) : 204 -209 . DOI: 10.6023/cjoc201307047
An efficient and easy method for the synthesis of coumarino[4,3-b]pyran-4-one derivatives underwent oxidative cyclization of 3-cinnamoyl-4-hydroxycoumarins, synthesized from condensation between 3-acetyl-4-hydroxycoumarin and aromatic aldehydes in the presence of piperidine and PEG-400 by using iodine in DMSO, has been described. All of above compounds have been confirmed by NMR, IR, MS spectra and elemental analysis.

[1] (a) Horton, D. A.; Bourne, G. T.; Smythe, M. L. Chem. Rev. 2003, 103, 893.
(b) Ma, W. H.; Xia, W.; Xu, Q.; Han, H. Y.; Song, B.; Sun, L. W.; Liang, C. H. Acta Chim. Sinica 2012, 70, 917 (in Chinese).
(马文辉, 夏威, 徐群, 韩宏彦, 宋波, 孙丽微, 梁春花, 化学学报, 2012, 70, 917.;
c) Jiang, J. Q.; Chen, X.; Yin, M.; Wang, Z. P.; Liu, X. Y.; Chen, M. Q. Acta Chim. Sinica 2012, 70, 525 (in Chinese).
(江金强, 陈欣, 殷明, 王周平, 刘晓亚, 陈明清, 化学学报, 2012, 70, 525.)
(d) Chen, Z. W.; Bi, J. H.; Su, W. K. Chin. J. Chem., 2013, 31, 507.
(e) Al-Sehemi, A. G.; El-Gogary S. R. Chin. J. Chem. 2012, 30, 316.
[2] Geen, G. R.; Evans, J. M.; Vong, A. K. In Comprehensive Heterocyclic Chemistry II, Pergamon, Oxford, 1984, 5, 469.
[3] (a) Audisio, D.; Messaoudi, S.; Brion, J.-D.; Alami, M. Eur. J. Org. Chem. 2010, 6, 1046.
(b) Khan, A. T.; Das, D. K. Tetrahedron Lett. 2012, 53, 2345.
[4] (a) Rahman, M. M.; Gray, A. I.; Khondkar, P.; Sarker, S. D. Pharm. Biol. 2008, 46, 356.
(b) Meragelman, T. L.; Tucker, K. D.; McClord, T. G.; Cardel-lina, J. H.; Shoemker, R. H. J. Nat. Prod. 2005, 68, 1790.
[5] Hirpara, K. V.; Aggarwal, P.; Mukherjee, A. J.; Joshi, N.; Burman, A. C. Curr. Med. Chem. 2009, 9, 138.
[6] Mustafa, A.; Hsihmat, O. H.; Zayed, S. M. A. D.; Nawar, A. A. Tetrahedron 1963, 19, 1831.
[7] Mulwad, V. V.; Pawar, R. B.; Chaskar, A. C. J. Korean Chem. Soc. 2008, 52, 249.
[8] Karia, D. C.; Pandya, H. K.; Godvani, N. K. Asian J. Biochem. Pharm. Res. 2012, 2, 126.
[9] Wang, D. L.; Li, D.; Cao, L. Chin. J. Org. Chem. 2012, 32, 1741 (in Chinese).
(王道林, 李帝, 曹亮, 有机化学, 2012, 32, 1741.)
[10] Wang, D. L.; Yu, J. Y.; Xu, J.; Dong, Z. Heterocyles 2013, 87, 1099.
[11] Wang, D. L.; Dong, Z.; Xu, J.; Li, D. Chin. J. Org. Chem. 2013, 33, 1559 (in Chinese).
(王道林, 董哲, 徐姣, 李帝, 有机化学, 2013, 33, 1559.)
[12] (a) Hamdi, N.; Bouabdallah, F.; Romerosa, A.; Benhassen, R. C. R. Chim. 2010, 13, 1261.
(b) Trivedi, J. C.; Bariwal, J. B.; Upadhyay, K. D.; Naliapara, Y. T.; Joshi, S. K.; Pannecouque, C. C.; Clercq, E. D.; Shah, A. K. Tetrahedron Lett. 2007, 48, 8472.
(c) Hamdi, N.; Fischmeister, C.; Puerta, M. C.; Valerga, P. Med. Chem. Res. 2011, 20, 522.
[13] Zhou, Y.; Yan, P. F.; Li, G. M.; Chen, Z. J. Chin. J. Org. Chem. 2009, 29, 1719 (in Chinese).
(周颖, 闫鹏飞, 李光明, 陈正军, 有机化学, 2009, 29, 1719.)
[14] (a) Miyake, H.; Takizawa, E.; Sasaki, M. Bull. Chem. Soc. Jpn. 2003, 76, 835.
(b) Lokhande, P. D.; Sakate, S. S.; Taksande, K. N.; Navghare, B. Tetrahedron Lett. 2005, 46, 1573.
(c) Maloney, D. J.; Hecht, S. M. Org. Lett. 2005, 7, 1097.
[15] Mavel. S.; Dikic, B.; Palakas, S.; Emond, P.; Greguric, I.; de Gracia, A. G.; Mattner, F.; Garrigos, M.; Guilloteau, D.; Katsifis, A. Bioorg. Med. Chem. 2006, 14, 1599.
[16] Santosusso, T. M.; Swern, D. J. Org. Chem. 1975, 40, 2764.
[17] Miyake, H.; Takizawa, E.; Sasaki, M. Bull. Chem. Soc. Jpn. 2003, 76, 835.
/
| 〈 |
|
〉 |