

多咪唑盐合成的N-杂环卡宾金属配合物研究进展
收稿日期: 2013-07-31
修回日期: 2013-10-03
网络出版日期: 2013-10-11
基金资助
国家自然科学基金(Nos.21172114,21172036)资助项目.
Recent Progress of N-Heterocyclic Carbene Complexes Synthesized by Poly-imidazolium Salts
Received date: 2013-07-31
Revised date: 2013-10-03
Online published: 2013-10-11
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21172114, 21172036).
林彩霞 , 郭琳 , 李庆山 , 张正之 , 袁耀锋 , 徐凤波 . 多咪唑盐合成的N-杂环卡宾金属配合物研究进展[J]. 有机化学, 2014 , 34(2) : 239 -266 . DOI: 10.6023/cjoc201307055
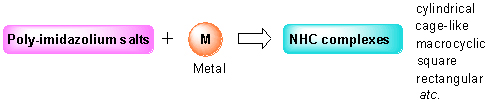
Owing to the excellent chemical stability and catalytic activity, N-heterocyclic carbene (NHC) complexes have always been the hotspot in the field of organic chemistry. Imidazolium salts are the precursors of NHCs, and their easy preparation and structure diversity properties make them useful for the generation of NHC complexes with topology structures. In this paper, the recent progress of synthesis, structure and physicochemical properties of NHC complexes with cylindrical, cage-like, macrocyclic, square and rectangular molecular structures which are synthesized by poly-imidazolium salts is summarized and reviewed.
[1] Herrmann, W. A. Angew. Chem., Int. Ed. 2002, 41, 1290.
[2] Kühl, O. Functionalised N-Heterocyclic Carbene Complexes, John Wiley & Sons Ltd., Chicester, 2010.
[3] Liu, Q-X.; Li, Z.-M. Chemistry 2004, 10, 715 (in Chinese). (柳清湘; 李正名, 化学通报, 2004, 10, 715.)
[4] (a) Hahn, F. E.; Jahnke, M. C. Angew. Chem., Int. Ed. 2008, 47, 3122. (b) de Frémont, P.; Marion, N.; Nolan, S. P. Coord. Chem. Rev. 2009, 253, 862.
[5] (a) Velazquez, H. D.; Verpoort, F. Chem. Soc. Rev. 2012, 41, 7032. (b) Nolan, S. P. Acc. Chem. Res. 2010, 44, 91. (c) Credendino, R.; Falivene, L.; Cavallo, L. J. Am. Chem. Soc. 2012, 19, 8127. (d) Zhang, R.; Xu, Q.; Shi, M. Acta Chim. Sinica 2012, 70, 1593 (in Chinese). (张睿; 徐琴; 施敏, 化学学报, 2012, 70, 1593.) (e) Cai, X.; Xie, B. Chin. Appl. Chem. 2013, 30, 123 (in Chinese). (蔡小华; 谢兵, 应用化学, 2013, 30, 123.) (f) Chen, Y.; Kong, L. Chin. J. Org. Chem. 2012, 32, 511 (in Chinese). (承勇; 孙礼林, 有机化学, 2012, 32, 511.) (g) He, T.; Wang, M.; Li, P.; Wang, L. Chin. J. Chem. 2012, 30, 979. (h) Zhang, R.; Wang, D.; Xu, Q.; Jiang, J.; Shi, M. Chin. J. Chem. 2012, 30, 1295. (i) Qu, M.-N.; He, J.-M. Chin. J. Org. Chem. 2011, 31, 1388 (in Chinese). (屈孟男, 何金梅, 有机化学, 2011, 31, 1388.)
[6] (a) Boydston, A. J.; Williams, K. A.; Bielawski, C. W. J. Am. Chem. Soc. 2005, 127, 12496. (b) Boydston, A. J.; Bielawski, C. W. Dalton Trans. 2006, 4073. (c) Tennyson, A. G.; Kamplain, J. W.; Bielawski, C. W. Chem. Commun. 2009, 2124. (d) Mercs, L.; Neels, A.; Stoeckli-Evans, H.; Albrecht, M. Dalton Trans. 2009, 7168.
[7] (a) Hindi, K. M.; Panzner, M. J.; Tessier, C. A.; Cannon, C. L.; Youngs, W. J. Chem. Rev. 2009, 109, 3859. (b) Ray, S.; Mohan, R.; Singh, J. K.; Samantaray, M. K.; Shaikh, M. M.; Panda, D.; Ghosh, P. J. Am. Chem. Soc. 2007, 129, 15042. (c) Hickey, J. L.; Ruhayel, R. A.; Barnard, P. J.; Baker, M. V.; Berners-Price, S. J.; Filipovska, A. J. Am. Chem. Soc. 2008, 130, 12570.
[8] Liu, Q.-X.; Li, Z.-M. Chem. Res. Appl. 2005, 17, 147 (in Chinese). (柳清湘; 李正名, 化学研究与应用, 2005, 17, 147.)
[9] Rit, A.; Pape, T.; Hepp, A.; Hahn, F. E. Organometallics 2011, 30, 334.
[10] (a) Mata, J. A.; Poyatos, M.; Peris, E. Coord. Chem. Rev. 2007, 251, 841. (b) Poyatos, M.; Mata, J. A.; Peris, E. Chem. Rev. 2009, 109, 3677.
[11] Baker, M. V.; Skelton, B. W.; White, A. H.; Williams, C. C. J. Chem. Soc., Dalton Trans. 2001, 111.
[12] Baker, M. V.; Brown, D. H.; Simpson, P. V.; Skelton, B. W.; White, A. H.; Williams, C. C. J. Organomet. Chem. 2006, 691, 5845.
[13] Baker, M. V.; Brayshaw, S. K.; Skelton, B. W.; White, A. H.; Williams, C. C. J. Organomet. Chem. 2005, 690, 2312.
[14] (a) Baker, M. V.; Brown, D. H.; Haque, R. A.; Skelton, B. W.; White, A. H. Dalton Trans. 2004, 3756. (b) Barnard, P. J.; Baker, M. V.; Berners-Price, S. J.; Skelton, B. W.; White, A. H. Dalton Trans. 2004, 1038.
[15] Fortman, G. C.; Nolan, S. P. Chem. Soc. Rev. 2011, 40, 5151.
[16] Lin, J. C. Y.; Huang, R. T. W.; Lee, C. S.; Bhattacharyya, A.; Hwang, W. S.; Lin, I. J. B. Chem. Rev. 2009, 109, 3561.
[17] Baker, M. V.; Brown, D. H.; Heath, C. H.; Skelton, B. W.; White, A. H.; Williams, C. C. J. Org. Chem. 2008, 73, 9340.
[18] Barnard, P. J.; Wedlock, L. E.; Baker, M. V.; Berners-Price, S. J.; Joyce, D. A.; Skelton, B. W.; Steer, J. H. Angew. Chem., Int. Ed. 2006, 45, 5966.
[19] Melaiye, A.; Sun, Z.; Hindi, K.; Milsted, A.; Ely, D.; Reneker, D. H.; Tessier, C. A.; Youngs, W. J. J. Am. Chem. Soc. 2005, 127, 2285.
[20] Radloff, C.; Gong, H.-Y.; Schulte to Brinke, C.; Pape, T.; Lynch, V. M.; Sessler, J. L.; Hahn, F. E. Chem.-Eur. J. 2010, 16, 13077.
[21] Loch, J. A.; Albrecht, M.; Peris, E.; Mata, J.; Faller, J. W.; Crabtree, R. H. Organometallics 2002, 21, 700.
[22] Garrison, J. C.; Simons, R. S.; Talley, J. M.; Wesdemiotis, C.; Tessier, C. A.; Youngs, W. J. Organometallics 2001, 20, 1276.
[23] Garrison, J. C.; Simons, R. S.; Tessier, C. A.; Youngs, W. J. J. Organomet. Chem. 2003, 673, 1.
[24] Baker, M. V.; Skelton, B. W.; White, A. H.; Williams, C. C. Organometallics 2002, 21, 2674.
[25] Wang, D.; Zhang, B.; He, C.; Wu, P.; Duan, C. Chem. Commun. 2010, 46, 4728.
[26] Hahn, F. E.; Radloff, C.; Pape, T.; Hepp, A. Chem.-Eur. J. 2008, 14, 10900.
[27] Schulte to Brinke, C.; Pape, T.; Hahn, F. E. Dalton Trans. 2013, 42, 7330.
[28] McKie, R.; Murphy, J.; Park, S.; Spicer, M.; Zhou, S.-z. Angew. Chem., Int. Ed. 2007, 46, 6525.
[29] Lu, Z.; Cramer, S. A.; Jenkins, D. M. Chem. Sci. 2012, 3, 3081.
[30] Hiraoka, S.; Shiro, M.; Shionoya, M. J. Am. Chem. Soc. 2004, 126, 1214.
[31] (a) Rit, A.; Pape, T.; Hahn, F. E. J. Am. Chem. Soc. 2010, 132, 4572. (b) Rit, A.; Pape, T.; Hahn, F. E. Organometallics 2011, 30, 6393.
[32] Maity, R.; Rit, A.; Schulte to Brinke, C.; Daniliuc, C. G.; Hahn, F. E. Chem. Commun. 2013, 49, 1011.
[33] Ahamed, B. N.; Dutta, R.; Ghosh, P. Inorg. Chem. 2013, 8, 4269.
[34] Dias, H. V. R.; Jin, W. Tetrahedron Lett. 1994, 35, 1365.
[35] Nakai, H.; Tang, Y.; Gantzel, P.; Meyer, K. Chem. Commun. 2003, 24.
[36] (a) Kernbach, U.; Ramm, M.; Luger, P.; Fehlhammer, W. P. Angew. Chem., Int. Ed. 1996, 35, 310. (b) Fränkel, R.; Kernbach, U.; Bakola-Christianopoulou, M.; Plaia, U.; Suter, M.; Ponikwar, W.; Nöth, H.; Moinet, C.; Fehlhammer, W. P. J. Organomet. Chem. 2001, 617~618, 530.
[37] Fränkel, R.; Birg, C.; Kernbach, U.; Habereder, T.; Nöth, H.; Fehlhammer, W. P. Angew. Chem., Int. Ed. 2001, 40, 1907.
[38] Tubaro, C.; Biffis, A.; Scattolin, E.; Basato, M. Tetrahedron 2008, 64, 4187.
[39] Hu, X. L.; Tang, Y. J.; Gantzel, P.; Meyer, K. Organometallics 2003, 22, 612.
[40] Hu, X.; Castro-Rodriguez, I.; Olsen, K.; Meyer, K. Organometallics 2004, 23, 755.
[41] Hu, X.; Castro-Rodriguez, I.; Meyer, K. Organometallics 2003, 22, 3016.
[42] Hu, X.; Castro-Rodriguez, I.; Meyer, K. J. Am. Chem. Soc. 2003, 125, 12237.
[43] Garrison, J. C.; Youngs, W. J. Chem. Rev. 2005, 105, 3978.
[44] (a) Quezada, C. A.; Garrison, J. C.; Panzner, M. J.; Tessier, C. A.; Youngs, W. J. Organometallics 2004, 23, 4846. (b) Matsumoto, K.; Matsumoto, N.; Ishii, A.; Tsukuda, T.; Hasegawa, M.; Tsubomura, T. Dalton Trans. 2009, 6795.
[45] Jean-Baptiste dit Dominique, F.; Gornitzka, H.; Hemmert, C. J. Organomet. Chem. 2008, 693, 579.
[46] Cure, J.; Poteau, R.; Gerber, I. C.; Gornitzka, H.; Hemmert, C. Organometallics 2012, 31, 619.
[47] Baron, M.; Tubaro, C.; Biffis, A.; Basato, M.; Graiff, C.; Poater, A.; Cavallo, L.; Armaroli, N.; Accorsi, G. Inorg. Chem. 2012, 51, 1778.
[48] (a) Liu, Q.-X.; Yang, X.-Q.; Zhao, X.-J.; Ge, S.-S.; Liu, S.-W.; Zang, Y.; Song, H.-b.; Guo, J.-H.; Wang, X.-G. CrystEngComm 2010, 12, 2245. (b) Liu, Q. X.; Zhang, W.; Zhao, X. J.; Zhao, Z. X.; Shi, M. C.; Wang, X. G. Eur. J. Org. Chem. 2013, 1253.
[49] Hemmert, C.; Poteau, R.; dit Dominique, F. J.-B.; Ceroni, P.; Bergamini, G.; Gornitzka, H. Eur. J. Inorg. Chem. 2012, 3892.
[50] Paulose, T. A. P.; Wu, S.-C.; Olson, J. A.; Chau, T.; Theaker, N.; Hassler, M.; Quail, J. W.; Foley, S. R. Dalton Trans. 2012, 41, 251.
[51] Gil-Rubio, J.; Cámara, V.; Bautista, D.; Vicente, J. Inorg. Chem. 2013, 7, 4071.
[52] Papini, G.; Pellei, M.; Lobbia, G. G.; Burini, A.; Santini, C. Dalton Trans. 2009, 6985.
[53] Wang, J.; Gao, L.; Gan, Z.; Meng, F. Chin. J. Org. Chem. 2008, 28, 775 (in Chinese). (王君文, 高林英, 甘志刚, 孟繁慧, 有机化学, 2008, 28, 775.)
[54] (a) Wang, J.-W.; Song, H.-B.; Li, Q.-S.; Xu, F.-B.; Zhang, Z.-Z. Inorg. Chim. Acta 2005, 358, 3653. (b) Wang, J.-W.; Li, Q.-S.; Xu, F.-B.; Song, H.-B.; Zhang, Z.-Z. Eur. J. Org. Chem. 2006, 2006, 1310.
[55] Wang, J.-W.; Gao, L.-Y.; Meng, F.-H.; Jiao, J.; Ding, L.-Y.; Zhang, L.-F. J. Inclusion Phenom. Macrocyclic Chem. 2012, 73, 119.
[56] (a) Liu, Q.-X.; Zhao, X.-J.; Wu, X.-M.; Guo, J.-H.; Wang, X.-G. J. Organomet. Chem. 2007, 692, 5671. (b) Liu, Q.-X.; Yu, J.; Zhao, X.-J.; Liu, S.-W.; Yang, X.-Q.; Li, K.-Y.; Wang, X.-G. CrystEngComm 2011, 13, 4086.
[57] (a) Jean-Baptiste dit Dominique, F.; Gornitzka, H.; Sournia-Saquet, A.; Hemmert, C. Dalton Trans. 2009, 340. (b) Chen, J. C. C.; Lin, I. J. B. J. Chem. Soc., Dalton Trans. 2000, 839.
[58] Haque, R. A.; Ghdhayeb, M. Z.; Salman, A. W.; Budagumpi, S.; Khadeer Ahamed, M. B.; Abdul Majid, A. M. S. Inorg. Chem. Commun. 2012, 22, 113.
[59] Iqbal, M. A.; Haque, R. A.; Budagumpi, S.; Khadeer Ahamed, M. B.; Abdul Majid, A. M. S. Inorg. Chem. Commun. 2013, 28, 64.
[60] Haque, R. A.; Iqbal, M. A.; Budagumpi, S.; Khadeer Ahamed, M. B.; Abdul Majid, A. M. S.; Hasanudin, N. Appl. Organomet. Chem. 2013, 27, 214.
[61] Raynal, M.; Cazin, C. S. J.; Vallee, C.; Olivier-Bourbigou, H.; Braunstein, P. Chem. Commun. 2008, 3983.
[62] (a) Haque, R. A.; Salman, A. W.; Guan, T. S.; Abdallah, H. H. J. Organomet. Chem. 2011, 696, 3507. (b) Salman, A. W.; Haque, R. A.; Budagumpi, S. Polyhedron 2012, 42, 18.
[63] (a) Liu, Q.-X.; Wang, H.; Zhao, X.-J.; Yao, Z.-Q.; Wang, Z.-Q.; Chen, A.-H.; Wang, X.-G. CrystEngComm 2012, 14, 5330. (b) Liu, Q.-X.; Zhao, L.-X.; Zhao, X.-J.; Zhao, Z.-X.; Wang, Z.-Q.; Chen, A.-H.; Wang, X.-G. J. Organomet. Chem. 2013, 731, 35.
[64] Liu, Q.-X.; Chen, A.-H.; Zhao, X.-J.; Zang, Y.; Wu, X.-M.; Wang, X.-G.; Guo, J.-H. CrystEngComm 2011, 13, 293.
[65] Saito, S.; Saika, M.; Yamasaki, R.; Azumaya, I.; Masu, H. Organometallics 2011, 30, 1366.
[66] Wan, X.; Xu, F.; Zhang, Z.; Song, H. Z. Anorg. Allg. Chem. 2011, 637, 34.
[67] Liu, Y.-S.; Wan, X.-J.; Xu, F.-B. Organometallics 2009, 28, 5590.
[68] Lin, C.-X.; Kong, X.-F.; Xu, F.-B.; Zhang, Z.-Z.; Yuan, Y.-F. Z. Anorg. Allg. Chem. 2013, 639, 881.
[69] Liu, Q.-X.; Yao, Z.-Q.; Zhao, X.-J.; Zhao, Z.-X.; Wang, X.-G. Organometallics 2013, 32, 3493.
[70] Han, Y.-F.; Jin, G.-X.; Hahn, F. E. J. Am. Chem. Soc. 2013, 135, 9263.
[71] Wan, X.-J.; Xu, F.-B.; Li, Q.-S.; Song, H.-B.; Zhang, Z.-Z. Inorg. Chem. Commun. 2005, 8, 1053.
[72] (a) Liu, Q.-X.; Yao, Z.-Q.; Zhao, X.-J.; Chen, A.-H.; Yang, X.-Q.; Liu, S.-W.; Wang, X.-G. Organometallics 2011, 30, 3732. (b) Wan, X.-J.; Xu, F.-B.; Li, Q.-S.; Song, H.-B.; Zhang, Z.-Z. Organometallics 2005, 24, 6066.
[73] Zhang, X.; Qiu, Y.; Rao, B.; Luo, M. Organometallics 2009, 28, 3093.
[74] Zhang, W.; Zhang, X.; Luo, M. Chin. J. Chem. 2012, 30, 1423.
[75] (a) Liu, N.; Liu, C.; Jin, Z. Chin. J. Org. Chem. 2012, 32, 860 (in Chinese). (刘宁; 刘春; 金子林, 有机化学, 2012, 32, 860.) (b) Karimi, B.; Akhavan, P. F. Chem. Commun. 2011, 47, 7686. (c) Fihri, A.; Luart, D.; Len, C.; Solhy, A.; Chevrin, C.; Polshettiwar, V. Dalton Trans. 2011, 40, 3116.
[76] (a) Liu, B.; Chen, W.; Jin, S. Organometallics 2007, 26, 3660. (b) Zhang, X.; Xi, Z.; Liu, A.; Chen, W. Organometallics 2008, 27, 4401.
[77] Gu, S.-J.; Huang, J.-J.; Chen, W.-Z. J. Org. Chem. 2013, 33, 713 (in Chinese). (顾绍金, 黄菁菁, 陈万芝, 有机化学, 2013, 33, 713.)
[78] Budagumpi, S.; Haque, R. A.; Salman, A. W.; Ghdhayeb, M. Z. Inorg. Chim. Acta 2013, 392, 61.
[79] Mokhtari, B.; Pourabdollah, K.; Dalali, N. J. Inclusion Phenom. Macrocyclic Chem. 2011, 69, 1.
[80] (a) Fahlbusch, T.; Frank, M.; Maas, G.; Schatz, J. Organometallics 2009, 28, 6183. (b) Frank, M.; Maas, G.; Schatz, J. Eur. J. Org. Chem. 2004, 607.
[81] Dinarès, I.; Garcia de Miguel, C.; Font-Bardia, M.; Solans, X.; Alcalde, E. Organometallics 2007, 26, 5125.
[82] (a) Lin, C.-X.; Kong, X.-F.; Li, Q.; Zhang, Z.-Z.; Yuan, Y.; Xu, F.-B. CrystEngComm 2013, 15, 6948. (b) Qin, D.-B.; Zeng, X.-X.; Li, Q.-X.; Xu, F.-B.; Song, H.-B.; Zhang, Z.-Z. Chem. Commun. 2007, 147.
[83] Hahn, F. E.; Radloff, C.; Pape, T.; Hepp, A. Organometallics 2008, 27, 6408.
[84] Radloff, C.; Weigand, J. J.; Hahn, F. E. Dalton Trans. 2009, 9392.
[85] Conrady, F. M.; Froehlich, R.; Brinke, C. S. T.; Pape, T.; Hahn, F. E. J. Am. Chem. Soc. 2011, 133, 11496.
/
| 〈 |
|
〉 |