

周环上芳基取代的叶绿素类二氢卟吩衍生物的合成
收稿日期: 2013-07-31
修回日期: 2013-10-11
网络出版日期: 2013-10-25
基金资助
国家自然科学基金(No.21272048)和山东省黄金工程技术研究中心(2011年度)资助项目.
Synthesis of Chlorophyllous Chlorins Derivatives Substituted by Aromatic Groups on Their Periphery
Received date: 2013-07-31
Revised date: 2013-10-11
Online published: 2013-10-25
Supported by
Project supported by the National Natural Science Foundations of China (No. 21272048) and the Project of Shandong Applied Research Centre of Gold Nanotechnology (2011).
于沙沙 , 徐希森 , 刘洋 , 李家柱 , 金英学 , 祁彩霞 , 王进军 . 周环上芳基取代的叶绿素类二氢卟吩衍生物的合成[J]. 有机化学, 2014 , 34(2) : 362 -370 . DOI: 10.6023/cjoc201307056
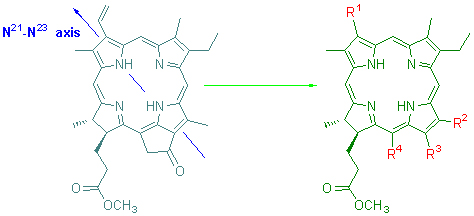
Pyropheophorbide-a methyl ester, as a degraded product from chlorophyll-a, was used as a starting material, and the modifications for functional group along N21-N23-axis and reconstructions for five-membered exocyclic E-ring were completed to introduce different aromatic groups which could conjugated with macrocyclic chromophore at various degrees on the periphery. The syntheses of 12 unreported chlorins related to chlorophyll were accomplished and their chemical structures were characterized by elemental analysis, UV, IR, MS and 1H NMR spectra. The different effects on the physical and chemical properties of chlorophyllous chlorins by introduction of aromatic groups were discussed.
[1] (a) Gil, M.; Bieniaszl, M.; Seshadri, M.; Fisher, D.; Ciesielski, M. J.; Chen, Y.; Pandey, R. K.; Kozbor, D. Brit. J. Cancer 2011, 103, 1.
(b) Bellnier, D. A.; Greco, W. R.; Loewen, G. M.; Nava, H.; Oseroff, A. R.; Pandey, R. K.; Tsuchida, T.; Dougherty, T. J. Cancer Res. 2003, 63, 1806.
[2] (a) Tamiaki, H.; Shibata, R.; Mizoguchi, T. Photochem. Photobiol. 2007, 83, 152.
(b) Goswami, L. N.; Ethirajan, M.; Dobhal, M. P.; Zhang, M.; Missert, J. R.; Shibata, M.; Kadish, K. M.; Pandey, R. K. J. Org. Chem. 2009, 74, 568.
[3] Hoober, J. K.; Eggink, L. L.; Chen, M. Photosynth. Res. 2007, 94, 387.
[4] (a) Ethirajan, M.; Joshi, P.; William, W. H.; Ohkubo, K.; Fukuzumi, S.; Pandey, R. K. Org. Lett. 2011, 8, 1956.
(b) Mennenga, A.; Wolfgang Gartner, W.; Lubitz, W.; Gorner, H. Phys. Chem. Chem. Phys. 2006, 8, 5444.
(c) Ethirajan, M.; Joshi, P.; William, W. H.; Ohkubo, K.; Fukuzumi, S.; Pandey, R. K. Org. Lett, 2011, 13, 1956.
[5] Chen, Y. H.; Li, G. L.; Pandey, R. K. Curr. Org. Chem. 2004, 8, 1105.
[6] (a) Washington, I.; Brooks, C.; Turro, N. J.; Nakanishi, K. J. Am. Chem. Soc. 2004, 126, 9892.
(b). Steruberg, E. D.; Dolphin, D. Tetrahedron 1998, 54, 415.
(c) Zheng, G.; Potter, W. R.; Camacho, S. H.; Missert, J. R.; Wang, G.-S.; Bellnier, D. A.; Henderson, B. W.; Rodgers, M. A. J.; Dougherty, T. J.; Pandey, R. K. J. Med. Chem. 2001, 44, 1540.
[7] (a) Wang, J. J.; Li, J.-J.; Wu, X.-R.; Shim, Y.-K. Chin. J. Chem. 2006, 24, 933.
(b) Wang, J.-J.; Li, J.-Z.; Li, Y.-W.; Jakus, J.; Shim, Y.-K. J. Porphyrins Phthalocyanines 2010, 14, 859.
(c) Ji, J.-Y.; Li, J.-Z.; Wang, H.; Li, F.-G.; Han, G.-F.; Shen, R.-K.; Wang, J.-J. Chin. J. Org. Chem. 2006, 26, 1714 (in Chinese).
(纪建业, 李家柱, 王虎, 李付国, 韩光范, 沈荣基, 王进军, 有机化学, 2006, 26, 1714.)
(d) Li, J.-Z.; Wang, J.-J.; Yoon, L.; Cui, B.-C.; Shim, Y.-K. Bioorg. Med. Chem. Lett. 2012, 22, 1846.
(e) Wang, J. J.; Liu, C.-L.; Li, J.-J. Synth. Commun. 2012, 42, 487.
(f) Wang, L.-M.; Wang, Z.; Yang. Z.; Jin, Y.-X.; Wang, J.-J. Chin. J. Org. Chem. 2012, 32, 2154 (in Chinese).
(王鲁敏, 王振, 杨泽, 金英学, 王进军, 有机化学, 2012, 32, 2154.)
(g) Wang, J.-J.; Li, F.-G.; Li, Y.-W. Chin. J. Org. Chem. 2011, 31, 68 (in Chinese).
(王进军, 李付国, 李韵伟, 有机化学, 2011, 31, 68.)
[8] Wang, J.-J. Chin. J. Org. Chem. 2005, 25, 1353 (in Chinese).
(王进军, 有机化学, 2005, 25, 1353.)
[9] Wang, J.-J.; Wu, X.-R.; Wang, L.-M.; Han, G.-F.; Shin, R.-K. Chin. J. Org. Chem. 2004, 24, 906 (in Chinese).
(王进军, 邬旭然, 王鲁敏, 韩光范, 沈荣基, 有机化学, 2004, 24, 906.)
[10] (a) Li, J.-J.; Liu, W.-H.; Li, F.-G.; Wang, J.-J.; Suo, Y.-R.; Liu, Y.-J. Chin. J. Org. Chem. 2007, 27, 1594 (in Chinese).
(李家柱, 刘万卉, 李付国, 王进军, 索有瑞, 刘永军, 有机化学, 2007, 27, 1594.)
(b) Tamoata, H.; Kouraba, M. Tetrahedron 1997, 53, 10677.
[11] (a) Li, J.-Z.; Wang, J.-J.; Yoon, L.; Cui, B.-C.; Shim, Y.-K. Bioorg. Med. Chem. Lett. 2012, 22, 1846.
(b) Wang, J.J.; Liu, C.-L.; Li, J.-J. Synth. Commun. 2012, 42, 487.
(c) Wang, J.-J.; Wang, P.; Li, J.-J.; Jakus, J.; Shim, Y.-K. Bull. Korean Chem. Soc. 2011, 32, 3473.
[12] Li, J.-Z. M.S. Thesis, Yantai University, Yantai, 2007 (in Chinese).
(李家柱, 硕士论文, 烟台大学, 烟台, 2007.)
[13] Wu, X.-R.; Liu, C.; Yang, Z.; Yao, N.-N.; Wang, J.-J. Chin. J. Org. Chem. 2012, 32, 632 (in Chinese).
(邬旭然, 刘超, 杨泽, 姚楠楠, 王进军, 有机化学, 2012, 32, 632.)
[14] Ji, J.-Y.; Xia, S.-W.; Zhao, L.-L.; Li, J.-Z.; Qi, C.-X.; Wang, J.-J. Chin. J. Org. Chem. 2013, 33, 1457 (in Chinese).
(纪建业, 夏尚文, 赵丽丽, 李家柱, 祁彩霞, 王进军, 有机化学, 2013, 33, 1457.)
[15] Smith, K. M.; Gogg, D. A.; Simpson, D. J. J. Am. Chem. Soc. 1985, 107, 4946.
[16] Wang, J.-J.; Li, Y.-W.; Li, J.-Z.; Yin, J.-G. Chin. J. Org. Chem. 2011, 31, 2074 (in Chinese).
(王进军, 李韵伟, 李家柱, 殷军港, 有机化学, 2011, 31, 2074.)
/
| 〈 |
|
〉 |