

新型双环新烟碱类似物的合成及其生物活性
收稿日期: 2013-08-26
修回日期: 2013-09-25
网络出版日期: 2013-10-28
基金资助
国家重点基础研究发展计划(973计划,No.2010CB126100)、国家高技术研究发展计划(863计划,No.2011AA10A207)、“十一五”国家科技支撑计划(No.2011BAE06B01)和国家自然科学基金(No.21272071)资助项目.
Synthesis and Insecticidal Evaluation of Neonicotinoid Analogues with Bicyclic Ring System
Received date: 2013-08-26
Revised date: 2013-09-25
Online published: 2013-10-28
Supported by
Project supported by the National Basic Research Program of China (973 Program, No. 2010CB126100), the National High Technology Research and Development Program of China (863 Program, No. 2011AA10A207), the National Key Technology R&D Program of China (No. 2011BAE06B01) and the National Natural Science Foundation of China (No. 21272071).
陈寅波 , 范杰 , 夏爽 , 程家高 , 徐晓勇 , 李忠 . 新型双环新烟碱类似物的合成及其生物活性[J]. 有机化学, 2014 , 34(2) : 409 -413 . DOI: 10.6023/cjoc201308033
Six novel neonicotinoid analogues with bicyclic ring system were designed and synthesized, and their structures were characterized by 1H NMR, 13C NMR, HRMS and single crystal X-ray diffraction analysis. Preliminary bioassays showed that five target compounds 6a, 6c~6f exhibited good insecticidal activities against cowpea aphids (Aphis craccivora) at 500 mg·L-1. Docking study was applied to investigate the effect of the bicyclic ring system of the target compound 6a on the biological activity, and compared with mode of action of imidacloprid (IMI) and compound 6a. It was found that the only π-π stacking interaction was found between the plane of pyridine of the target compounds 6a and the aromatic side chain of Tyr 147, which was different binding mode with that of IMI.
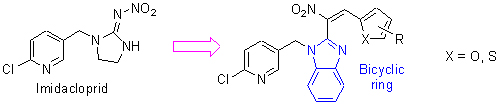
Key words: neonicotinoid analogue; synthesis; bioactivity; crystal structure; molecular docking
[1] Jeschke, P.; Nauen, R. Pest. Manage. Sci. 2008, 64, 1084.
[2] Kagabu, S. J. Agric. Food Chem. 2011, 59, 2887.
[3] Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. J. Agric. Food Chem. 2011, 59, 2897.
[4] Tomizawa, M.; Kagabu, S.; Casida, J. E. J. Agric. Food Chem. 2011, 59, 2918.
[5] Shao, X. S.; Lee, P. W.; Liu, Z. W.; Xu, X. X.; Li, Z.; Qian, X. H. J. Agric. Food Chem. 2011, 59, 2943.
[6] Tomizawa, M.; Maltby, D.; Talley, T. T.; Durkin, K. A.; Medzihradszky, K. F.; Burlingame, A. L.; Taylor, P.; Casida, J. E. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 1728.
[7] Matsuda, K.; Shimomura, M.; Ihara, M.; Akamatsu, M.; Sattelle, D. B. Biosci. Biotechnol. Biochem. 2005, 69, 1442.
[8] Tomizawa, M.; Casida, J. E. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 247.
[9] Xue, S. J.; Bu, H. F.; Liu, L.; Xu, X.; Ma, X. B. Chin. J. Chem. 2011, 29, 1011.
[10] Sun, C. W.; Chen, Y. X.; Liu, T. Y.; Wu, Y.; Fang, T.; Wang, J.; Xing, J. H. Chin. J. Chem. 2012, 30, 1415.
[11] Wang, B. Z.; Cheng, J. G.; Xu, Z. P.; Xu, X. X.; Shao, X. S.; Li, Z. Molecules 2012, 17, 10014.
[12] Zapolskii, V. A.; Fischer, R.; Namyslo, J. C.; Kaufmann, D. E. Bioorg. Med. Chem. 2009, 17, 4206.
[13] Novák, L.; Hornyánszkya, G.; Királya, I.; Rohálya, J.; Kolonitsa, P.; Szántaya, C. Heterocycles 2001, 55, 45.
[14] Tomizawa, M.; Yamamoto, I. Nihon Noyaku Gakkaishi 1993, 18, 91.
[15] Kagabu, S.; Matsuno, H. J. Agric. Food Chem. 1997, 45, 276.
[16] Tomizawa, M.; Zhang, N.; Durkin, K. A.; Olmstead, M. M.; Casida, J. E. Biochemistry 2003, 42, 7819.
[17] Wang, Y. L.; Cheng, J. G.; Qian, X. H.; Li, Z. Bioorg. Med. Chem. 2007, 15, 2624.
[18] Tomizawa, M.; Talley, T. T.; Maltby, D.; Durkin, K. A.; Medzihradszky, K. F.; Burlingame, A. L.; Taylor, P.; Casida, J. E. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 9075.
[19] Ihara, M.; Okajima, T.; Yamashita, A.; Oda, T.; Hirata, K.; Nishiwaki, H.; Morimoto, T.; Akamatsu, M.; Ashikawa, Y.; Kuroda, S.; Mega, R.; Kuramitsu, S.; Sattelle, D. B.; Matsuda, K. Invert. Neurosci. 2008, 8, 71.
[20] Shao, X. S.; Li, Z.; Qian, X. H.; Xu, X. Y. J. Agric. Food Chem. 2009, 57, 951.
[21] Zhang, W. W.; Yang, X. B.; Chen, W. D.; Xu, X. Y.; Li, L.; Zhai, H. B.; Li, Z. J. Agric. Food Chem. 2010, 58, 2741.
[22] Ye, Z. J.; Xia, S.; Shao, X. S.; Cheng, J. G.; Xu, X. Y.; Xu, Z. P.; Li, Z.; Qian, X. H. J. Agric. Food Chem. 2011, 59, 10615.
[23] Shao, X. S.; Xu, Z. P.; Zhao, X. F.; Xu, X. Y.; Tao, L. M.; Li, Z.; Qian, X. H. J. Agric. Food Chem. 2010, 58, 2690.
[24] Tomizawa, M.; Casida, J. E. J. Agric. Food Chem. 2011, 59, 2825.
[25] Tomizawa, M.; Casida, J. E. Acc. Chem. Res. 2009, 42, 260.
[26] Talley, T. T.; Harel, M.; Hibbs, R. E.; Radic, Z.; Tomizawa, M.; Casida, J. E.; Taylor, P. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 7606.
/
| 〈 |
|
〉 |