

含酰亚胺骨架的大环化合物合成、结构及其对阴离子的识别研究
收稿日期: 2013-07-21
修回日期: 2013-09-27
网络出版日期: 2013-10-31
基金资助
国家自然科学基金(No.21061003)和贵州省国际合作基金[No.(2009)700104]资助项目.
Synthesis, Structures of Macrocyclic Compounds Containing Imide Skeleton and the Study on Recognition for F- Ion
Received date: 2013-07-21
Revised date: 2013-09-27
Online published: 2013-10-31
Supported by
Project supported by the National Natural Science Foundation of China (No. 21061003) and the International Cooperation Foundation of Guizhou Province [No. (2009)700104].
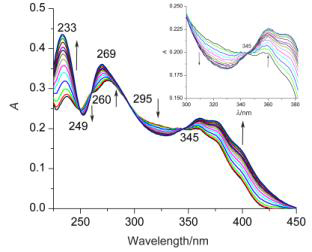
在磷酸催化作用下,采用前体二胺N,N'-(2-胺基苯基)-2,6-二甲酰亚胺吡啶(1)和前体二醛1,4-二(2'-甲酰苯氧基)丁烷(2)进行缩合作用得到[1+1] Schiff碱大环化合物3,进一步将Schiff碱大环3还原得到饱和大环4. 并采用1H NMR,IR,质谱和元素分析等技术对大环3和4的组成进行了表征. 采用X射线单晶衍射技术测定了Schiff碱大环3的晶体结构,结果表明大环 3 具有扭曲的“8”字形结构. 采用UV-vis光谱滴定技术对大环与系列阴离子的键合作用进行了考察,结果表明,Schiff碱大环3对F-离子有明显的选择性识别作用,并测定了该配位反应的配位比和平衡常数.
王芳芳 , 欧敏 , 邓雅欣 , 冉旭 , 张奇龙 , 朱必学 . 含酰亚胺骨架的大环化合物合成、结构及其对阴离子的识别研究[J]. 有机化学, 2014 , 34(2) : 334 -339 . DOI: 10.6023/cjoc201307033
A novel [1+1] Schiff base macrocyclic compound 3 has been synthesized from precursors N,N'-(2-amino- phenyl)pyridine-2,6-dicarboxamide (1) and 1,2-bis(2'-formacylphenoxy)tetrane (2) via condensation in the presence of the phosphoric acid. Furthermore the macrocycle 3 was reduced to the corresponding saturated macrocycle 4. Both macrocycles were characterized by 1H NMR, IR, MS and elemental analysis. The crystal structure of 3 was determined by X-ray diffraction analysis. The result reveals that the macrocycle 3 exhibits twisted to “figure eight” conformation. The results show that the macrocycle 3 displays a selective recognition ability for F- ion by the both macrocycles with a series of anions using UV-vis absorption spectra technique. The stoichiometric ratio and the stability constant of the coordination reaction were determined.

Key words: macrocyclic compound; synthesis; crystal structure; recognition
[1] Gale, P. A.; García-Garrido, S. E.; Garric, J. Chem. Soc. Rev. 2008, 37, 151.
[2] Kubik, S. Chem. Soc. Rev. 2009, 38, 585.
[3] Dorazco-González, A.; Höpfl, H.; Medrano, F.; Yatsimirsky, A. K. J. Org. Chem. 2010, 75, 2259.
[4] Sessler, J. L.; Katayev, E.; Pantos, G. D.; Ustynyuk, Y. A. Chem. Commun. 2004, 1276.
[5] Li, F.; Delgado, R.; Costa, J.; Drew, M. G. B.; Félix, V. Dalton Trans. 2005, 82.
[6] Hossain, M. A.; Kang, S. O.; Powell, D.; Bowman-James, K. Inorg. Chem. 2003, 42, 1397.
[7] Hu, P.; Guo, S.-Y.; Zhang, Q.-L.; Zhang, Y.-Q.; Zhu, B.-X. Chin. J. Org. Chem. 2013, 33, 325 (in Chinese).
(胡鹏, 郭思颖, 张奇龙, 张云黔, 朱必学, 有机化学, 2013, 33, 325.)
[8] Sheldrick, G. M. SHELX-97, University of Göttingen, Germany, 1997.
[9] Sellamuthu, A.; Kamalraj, S.; Varghese, B.; Johnpaul, M.; Muthusamy, K. Inorg. Chem. 2012, 51, 5580.
[10] Van Veggel, F. C. J. M.; Harkema, S.; Bas, M.; Verboom, W.; Van Staveren, C. J.; Gerritsma, G. J.; Reinhoudt, D. N. Inorg. Chem. 1989, 28, 1133.
[11] Van Staveren, C. J.; Van Eerden, J.; Van Veggel, F. C. J. M.; Harkema, S.; Reinhoudt, D. N. J. Am. Chem. Soc. 1988, 110, 4994.
[12] Ravikumar, I.; Pradyut, G. Chem. Soc. Rev. 2012, 41, 3077.
[13] Elwahy, A. H. M.; Masaret, G. S. J. Heterocycl. Chem. 2007, 44, 147.
[14] Zhu, B.-X.; Ruan, W.-J.; Yuan, R.-J.; Cao, X.-H.; Zhu, Z.-A. Chin. J. Appl. Chem. 2004, 10, 21 (in Chinese).
(朱必学, 阮文娟, 袁瑞娟, 曹小辉, 朱志昂, 应用化学, 2004, 10, 21.)
[15] Özkar, S.; Ü?kü, D.; Y?ld?r?m, L. T.; Biricik, N.; Gümgüm, B. J. Mol. Struct. 2004, 688, 207.
[16] Ou, M.; Deng, Y.-X.; Wang, F.-F.; Zhu, C.; Zhang, Q.-L.; Zhu, B. X. Chin. J. Org. Chem. 2013, 33, 1798 (in Chinese).
(欧敏, 邓雅欣, 王芳芳, 朱纯, 张奇龙, 朱必学, 有机化学, 2013, 33, 1798.)
/
| 〈 |
|
〉 |