

联吡啶金属配合物在偶联反应中的应用
收稿日期: 2013-09-27
修回日期: 2013-11-19
网络出版日期: 2013-12-13
基金资助
山西省自然科学基金(Nos. 2012021007-2,2011011010-2)及山西省高等教育机构科技创新项目基金(No. 20120006)资助项目.
Application of Bipyridyl Complexes in Coupling Reactions
Received date: 2013-09-27
Revised date: 2013-11-19
Online published: 2013-12-13
Supported by
Project supported by the Natural Science Foundation of Shanxi Province (Nos. 2012021007-2, 2011011010-2) and the Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi Province (No. 20120006).
李英俊 , 李志朋 , 李兴 , 常宏宏 , 魏文珑 . 联吡啶金属配合物在偶联反应中的应用[J]. 有机化学, 2014 , 34(4) : 693 -705 . DOI: 10.6023/cjoc201309039
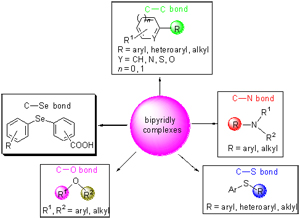
The bipyridyl ligands have been extensively used as metal chelating complexes or directly utilized as ligands due to their robust redox stability and ease of functionalization which also become more and more important in the application of the organic synthesis. This article mainly describes the application of bipyridyl complexes in coupling reactions for the formation of C—C, C—N, C—S, C—O and C—Se bonds.
[1] Kawano, T.; Shinomaru, T.; Ueda, I. Org. Lett. 2002, 4, 2545.
[2] Sinner, F.; Buchmeiser, M. R.; Tessadri, R.; Mupa, M.; Wurst, K.; Bonn, G. K. J. Am. Chem. Soc. 1998, 120, 2790.
[3] Kaes, K.; Katz, K.; Hosseini, W. Chem. Rev. 2000, 100, 3553.
[4] Beccalli, E. M.; Broggini, G.; Martinelli, M.; Sottocornola, S. Chem. Rev. 2007, 107, 5318.
[5] Turner, N. J. Chem. Rev. 2011, 111, 4073.
[6] Beletskaya, I. P.; Ananikov, V. P. Chem. Rev. 2011, 111, 1596.
[7] Ullmann, F. Ber. Dtsch. Chem. Ges. 1903, 36, 2382.
[8] Zhang, B. B.; Zhan, D.; Zhang, X. P.; Xiang, Q. J.; Zeng, Q. L. Acta Chim. Sinica 2012, 70, 1655 (in Chinese). (张斌彬, 詹丹, 张小平, 向沁洁, 曾庆乐, 化学学报, 2012, 70, 1655.)
[9] Li, X.; Yan, X. Y.; Chang, H. H.; Wang, L. C.; Zhang, Y.; Chen, W. W.; Li, Y. W.; Wei, W. L. Org. Biomol. Chem. 2012, 10, 495.
[10] Li, X.; Wang, L. C.; Chang, H. H.; Zhang, C. X.; Wei, W. L. Appl. Catal. A: Gen. 2013, 462~463, 15.
[11] Yin, J.; Rainka, M. P.; Zhang, X. X.; Buchwald, S. L. J. Am. Chem. Soc. 2002, 124, 1162.
[12] Matthias, A.; Oberli, M. A.; Stephen, L.; Buchwald, S. L. Org. Lett. 2012, 14, 4606.
[13] Jiang, L.; Li, Z. N.; Zhao, D. F. Acta Chim. Sinica 2010, 30, 200 (in Chinese). (姜岚, 李争宁, 赵德峰, 化学学报, 2012, 30, 200.)
[14] Paddock, R. L.; Nguyen, S. T. J. Am. Chem. Soc. 2001, 123, 11498.
[15] Mizoroki, T.; Mori, K.; Ozaki, A. Bull. Chem. Soc. Jpn. 1971, 44, 581.
[16] Heck, R. F.; Nolley, J. P. J. Org. Chem. 1972, 14, 2320
[17] Nájera, C.; Gil-Moltó, J.; Karlstrёm, S.; Falvello, L. R. Org. Lett. 2003, 5, 1451.
[18] Tsai, F. Y.; Wu, C. L.; Mou, C. Y.; Chao, M. C.; Lin, H. P.; Liu, S. T. Tetrahedron Lett. 2004, 45, 7503.
[19] Gil-Moltó, J.; Karlstrёm, S.; Nájera, C. Tetrahedron 2005, 61, 12168.
[20] Huang, S. H.; Chen, J. R.; Tsai, F. Y. Molecules 2010, 15, 315.
[21] Jatap, S. V.; Deshpande, R. M. Kinet. Catal. 2013, 54, 314.
[22] Miyauar, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457.
[23] Suzuki, A. Organomet. Chem. 1999, 576, 147.
[24] Nájera, C.; Gil-Moltó, J.; Karlstrom, S. Adv. Synth. Catal. 2004, 346, 1798.
[25] Wu, W. Y.; Chen, S. N.; Tsai, F. Y. Tetrahedron Lett. 2006, 47, 9267.
[26] Osako, T.; Uozumi, Y. Heterocycles 2010, 80, 505.
[27] Huang, J. P.; Wang, W.; Li, H. X. ACS Catal. 2013, 3, 1526.
[28] Negishi, E. I.; Baba, S. J. Chem. Soc., Chem. Commun. 1976, 15, 596.
[29] Baba, S.; Negishi, E. I. J. Am. Chem. Soc. 1976, 98, 6729.
[30] Wu, W. Y.; Lin, T. C.; Takahashi, T.; Tsai, F. Y.; Mou, C. Y. ChemCatChem 2013, 5, 1011.
[31] Hatanaka, Y.; Hiyama, T. J. Org. Chem. 1988, 53, 918.
[32] Chen, S. N.; Wu, W. Y.; Tsai, F. Y. Tetrahedron 2008, 64, 8164.
[33] Corriu, R. J. P.; Masse, J. P. J. Chem. Soc., Chem. Commun. 1972, 3, 144.
[34] Tamao, K.; Sumitani, K.; Kumada, M. J. Am. Chem. Soc. 1972, 94, 4374.
[35] Tsai, F. Y.; Lin, B. N.; Chen, M. J.; Mou, C. Y.; Liu, S. T. Tetrahedron 2007, 63, 4304.
[36] Sonogashira, K. Organomet. Chem. 2002, 653, 46.
[37] Gil-Moltó, J.; Nájera, C. Eur. J. Org. Chem. 2005, 19, 4073.
[38] Lin, B. N.; Huang, S. H.; Wu, W. Y.; Mou, C. Y.; Tsai, F. Y. Molecules 2010, 15, 9157.
[39] Hung, T. T.; Huang, C. M.; Tsai, F. Y. ChemCatChem 2012, 4, 540.
[40] Fang, J. H.; Hu, M. J.; Wang, J. R.; Fu, Z. Q. J. Mol. Catal. 2006, 20, 335 (in Chinese). (房江华, 胡敏杰, 王家荣, 付志强, 分子催化, 2006, 20, 335.)
[41] Wang, Y. H.; Tsai, F. Y. Chem. Lett. 2007, 36, 1492.
[42] Chen, S. N.; Wu, W. Y.; Tsai, F. Y. Green Chem. 2009, 11, 269.
[43] Wu, T. M.; Huang, S. H.; Tsai, F. Y. Appl. Organomet. Chem. 2011, 25, 395.
[44] Yamamoto, T. Chem. Lett. 2012, 41, 1422.
[45] Chen, J. Y.; Chen, S. C.; Tang, Y. J.; Mou, C. Y.; Tsai, F. Y. J. Mol. Catal. A: Chem. 2009, 307, 88.
[46] Chen, J. Y.; Lin, T. C.; Chen, S. C.; Chen, A. J.; Mou, C. Y.; Tsai, F. Y. Tetrahedron 2009, 65, 10134.
[47] Nishihara, Y.; Ogawa, D.; Noyori, S.; Iwasaki, M. Chem. Lett. 2012, 41, 1503.
[48] Kohls, P.; Jadhav, D.; Pandey, G.; Reiser, O. Org. Lett. 2012, 14, 672.
[49] Savmarker, J.; Rydfjord, J.; Gising, J.; Odell, L. R.; Larhed, M. Org. Lett. 2012, 14, 2393.
[50] Yasu, Y.; Koike, T.; Akita, M. Chem. Commun. 2012, 48, 5355.
[51] Gao, X. W.; Meng, Q. Y.; Xing, M.; Chen. B.; Feng, K.; Tung, C. H.; Wu, L. Z. Adv. Synth. Catal. 2013, 355, 2158.
[52] Yi, C. Q.; Cheng, J.; Zhang, P. Chen, K. J. Wuhan Inst. Technol. 2013, 35, 1 (in Chinese). (尹传奇, 成军, 张平, 陈阔, 武汉工程大学学报, 2013, 35, 1.)
[53] Liang, L.; Li, Z. K.; Zhou, X. G. Org. Lett. 2009, 11, 3294.
[54] Srimani, D.; Balaraman, E.; Hu, P.; Yehoshoa, B. D.; Milstein, D. Adv. Synth. Catal. 2013, 355, 2525.
[55] Lanke, S. R.; Bhanage, B. M. Appl. Organomet. Chem. 2013, 27, 729.
[56] Wu, W. Y.; Wang, J. C.; Tsai, F. Y. Green Chem. 2009, 11, 326.
[57] Lan, M. T.; Wu, W. Y.; Huang, S. H.; Luo, K. L.; Tsai, F. Y. RSC Adv. 2011, 1, 1751.
[58] Wang, X.; Cuny, G. D.; Noёl, T. Angew. Chem., Int. Ed. 2013, 52, 7860.
[59] Koike, T.; Akita, M. Chem. Lett. 2009, 38, 166.
[60] Phan, N. T. S.; Vu, P. H. L.; Nguyen, T. T. J. Catal. 2013, 306, 38.
[61] Phan, N. T. S.; Nguyen, T. T.; Vu, P. H. L. ChemCatChem 2013, 5, 3068. Millois, C.; Diaz, P. Org. Lett. 2000, 2, 1705.
/
| 〈 |
|
〉 |