

查尔酮Mannich碱衍生物的合成与AChE抑制活性研究
收稿日期: 2013-11-14
修回日期: 2013-12-18
网络出版日期: 2013-12-23
基金资助
湖南省自然科学基金(No. 14JJ2048)和国家自然科学基金(No. 21342015)资助项目
Synthesis and AChE Inhibitory Activity of Chalcones Mannich Base Derivatives
Received date: 2013-11-14
Revised date: 2013-12-18
Online published: 2013-12-23
Supported by
Project supported by the Hunan Provincial Natural Science Foundation of China (No. 14JJ2048) and the National Natural Science Foundation of China (No. 21342015).
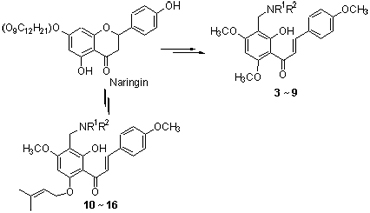
以柚皮苷为原料,经酸催化水解、O-甲基化或O-异戊烯基化反应、碱催化的开环反应合成了天然查尔酮卡瓦胡椒素A (1)和查尔酮异戊烯基醚衍生物2;然后以查尔酮1和2为底物,分别通过Mannich反应对其3’位进行了胺甲基化修饰,合成了14个未见文献报道的新型查尔酮Mannich碱衍生物3~16. 所合成化合物的结构已由核磁共振谱、红外光谱和质谱所证实,并对所合成的查尔酮及其Mannich碱衍生物进行了乙酰胆碱酯酶(AChE)抑制活性测试,结果发现查尔酮Mannich碱衍生物3~5,9具有良好的AChE抑制活性.
关键词: 查尔酮; 卡瓦胡椒素A; Mannich碱; 合成; 乙酰胆碱酯酶抑制活性
许守慧 , 刘浩然 , 娄定辉 , 汪秋安 . 查尔酮Mannich碱衍生物的合成与AChE抑制活性研究[J]. 有机化学, 2014 , 34(4) : 749 -755 . DOI: 10.6023/cjoc201311025
Chalcone flavokawain A (1) and chalcone prenyl ether derivative 2 were synthesized from naringin, through glycoside hydrolysis, O-methylation or O-prenylation, and base-catalyzed ring-opening reaction. Based on Mannich reaction of chalcone 1 and chalcone prenyl ether derivative 2, fourteen new chalcone Mannich base derivatives 3~16 were synthesized. The aminomethylation occurred preferentially at 3'-C position of chalcones. The structures of all synthesized compounds were determined by MS, NMR and IR spectra. All the synthetic compounds were evaluated for acetylcholinesterase (AChE) inhibitory activity. The results show that chalcone mannich base derivatives 3~5, 9 exhibit good AChE inhibitory activity.

/
| 〈 |
|
〉 |