

含咔唑基苯并咪唑衍生物的合成
收稿日期: 2013-11-13
修回日期: 2014-01-20
网络出版日期: 2014-01-24
基金资助
广东省大学生创新(No. 1055912003)资助项目.
Syntheses of Benzoimidazole Derivatives Containing Carbazole Unit
Received date: 2013-11-13
Revised date: 2014-01-20
Online published: 2014-01-24
Supported by
Project supported by the University Student Innovative Program of Guangdong Province (No. 1055912003).
卞垒 , 曾向潮 , 何如 , 罗创龙 , 林志强 . 含咔唑基苯并咪唑衍生物的合成[J]. 有机化学, 2014 , 34(5) : 994 -998 . DOI: 10.6023/cjoc201311018
Two series of novel benzoimidazole derivatives containing carbazole unit were synthesized via condensation-cyclization of N-alkylcarbazole-3-formaldehyde with 1,2-diaminobenzene in "one pot" reaction without catalyst. The effects of temperature, selection & amount of solvents and molar ratio of the two reagents on the reactions have been investigated; significantly, one of these two series of benzoimidazole derivatives could be prepared as the major products selectively by changing the molar ratio of the two reagents.
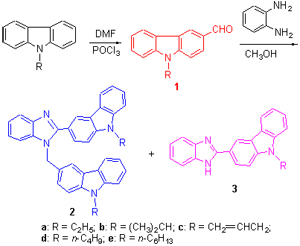
Key words: benzoimidazole; carbazole; organic synthesis
[1] Singh, M.; Tandon, V. Eur. J. Med. Chem. 2011, 46, 659.
[2] Zhan, P.; Chen, X. W.; Li, D. Y.; Fang, Z. J.; De Clercq, E.; Liu, X. Y. Med. Chem. Comm. 2013, 33, S1, E1.
[3] Sharma, M. C.; Kohli, D.; Sharma, S.; Sharma, A. D. Pharma Sin. 2010, 1, 104.
[4] Takahashi, K.; Hashimoto, N.; Nakama, C.; Kamata, K.; Sasaki, K.; Yoshimoto, R.; Ohyama, S.; Hosaka, H.; Maruki, H.; Nagata, Y.; Eiki, J.; Nishimura, T. Bioorg. Med. Chem. 2009, 17, 7042.
[5] Meng, J.-P.; Geng, R.-X.; Zhou, C.-H.; Gan, L.-L. Chin. J. New Drugs 2009, 18, 1505 (in Chinese).
(孟江平, 耿蓉霞, 周成合, 甘淋玲, 中国新药杂志, 2009, 18, 1505.)
[6] Peng, P.; Xiong, J.-F.; Li, B.; Mo, G.-Z.; Chen, R.-H.; Wang, Z.-Y. Chin. J. Org. Chem. 2013, 33, 1891 (in Chinese).
(彭湃, 熊金锋, 李豹, 莫广珍, 陈任宏, 汪朝阳, 有机化学, 2013, 33, 1891.)
[7] Batista, R. M. F.; Costa, S. P. G.; Raposo, M. M. M. J. Photochem. Photobiol., A: Chem. 2013, 259, 33.
[8] Park, S.; Kwon, O. H.; Kim, S.; Park, S.; Choi, M. G.; Cha, M.; Park, S. Y.; Jang, D. J. J. Am Chem. Sci. 2005, 127, 10070.
[9] Zhang, R.; Li, J.-T.; Fu, C.-L.; Luo, X.-T. Mater. Rev. 2011, 25, 61 (in Chinese).
(张蓉, 李锦堂, 傅翠梨, 罗学涛, 材料导报, 2011, 25, 61.)
[10] Wu Y.-F.; Cui, Y.-N.; Li, S.-M.; Jia, Y.-P.; Yin, J.-M. Chin. J. Inorg. Chem. 2012, 28, 910 (in Chinese).
(吴玉防, 崔颖娜, 李慎敏, 贾颖萍, 尹静梅, 无机化学学报, 2012, 28, 910.)
[11] Pina, J.; De Melo, J. S. S.; Batista, R. M. F.; Costa, S. P. G.; Raposo, M. M. M. J. Org. Chem. 2013, 78, 11389.
[12] Liu, L-T.; Ishida, N.; Ashida, S.; Murakami, M. Org. Lett. 2011, 13, 1666.
[13] Yu, K.; Guan, S.-X.; Zhang, H.-W.; Zhou, B.-B.; Li, L. Nat. Sci. J. Harbin Normal Univ. 2006, 22, 70 (in Chinese).
(于凯, 关淑霞, 张宏伟, 周百斌, 李玲, 哈尔滨师范大学自然科学学报, 2006, 22, 70.)
[14] Huang, B.; Tang, J.-N.; Jiang, W.; Yang, W.; Ban, X.-X.; Sun, Y.-M. Chin. J. Org. Chem. 2013, 33, 1395 (in Chinese).
(黄斌, 唐霁楠, 蒋伟, 杨文, 班鑫鑫, 孙岳明, 有机化学, 2013, 33, 1395.)
[15] Boufatah, N.; Gellis, A.; Maldonado, J.; Vanelle, P. Tetrahedron 2004, 60, 9131.
[16] Lu, J.; Ge, H.-G.; Bai, Y.-J. Chin. J. Org. Chem. 2002, 22, 782 (in Chinese).
(路军, 葛红光, 白银娟, 有机化学, 2002, 22, 782.)
[17] Lee, K. J.; Janda, K. D. Can. J. Chem. 2001, 79, 1556.
[18] Das, B.; Harish, H.; Srinivas, Y. Tetrahedron Lett. 2007, 48, 61.
[19] Chari, M. A.; Shobha, D.; Sasaki, T. Tetrahedron Lett. 2011, 52, 5575.
[20] Bahrami, K.; Khodaei, M. M.; Naali, F. J. Org. Chem. 2008, 73, 6835.
[21] Chakrabarty, M.; Mukherjee, R.; Karmakar, S.; Harigaya, Y. Monatsh. Chem. 2007, 138, 1279.
[22] Varala, R.; Nasreen, A.; Ramu, E.; Adapa, S. R. Tetrahedron Lett. 2007, 48, 69.
[23] Yang, H.-W.; Yue, F.; Feng, S.; Wang, J.-D.; Liu, A.-H.; Chen, H.-M.; Yu, K.-B. Chin. J. Org. Chem. 2004, 24, 792 (in Chinese).
(杨红伟, 岳凡, 封顺, 王吉德, 刘爱华, 陈华梅, 郁开北, 有机化学, 2004, 24, 792.)
[24] Lin, S.-N.; Yang, L.-H. Tetrahedron Lett. 2005, 46, 4315.
[25] Cui, L.-J.; Xiao, S.-Y.; Yang, H.-S.; Xu, P.; Cheng, F.-S.; Li, Z.-H.; Liang, R.-H.; Xia, Z.-N. Chin. J. Org. Chem. 2011, 31, 672 (in Chinese).
(崔丽君, 肖尚友, 杨昊书, 徐盼, 程凡圣, 李正华, 梁荣辉, 夏之宁, 有机化学, 2011, 31, 672.)
/
| 〈 |
|
〉 |