

收稿日期: 2013-12-22
修回日期: 2014-01-13
网络出版日期: 2014-02-10
基金资助
国家自然科学基金(No. 21172257)和十二五科技支撑计划(Nos. 2012BAK25B03-01,2011AA10A206)资助项目.
Efficient Synthesis of Allolactose
Received date: 2013-12-22
Revised date: 2014-01-13
Online published: 2014-02-10
Supported by
Project supported by the National Natural Science Foundation of China (No. 21172257) and the National S&T Pillar Program of China (Nos. 2012BAK25B03-01, 2011AA10A206).
姜锐 , 梁晓梅 , 金淑慧 , 张建军 , 王道全 . 异乳糖的合成研究[J]. 有机化学, 2014 , 34(5) : 926 -932 . DOI: 10.6023/cjoc201312028
Allolactose (β-D-galactopyranosyl-(1→6)-α,β-D-glucopyranose) is a reducing disaccharide playing important biological role in bacterial metabolism. Two synthetic methods of allolactose including regioselective glycosylation and stepwise synthesis were investigated using D-glucose and D-galactose as the starting materials. The later approach involved a five-spot strategy, 44% overall yields and all the intermediates were easily to be purified, making the route suitable for large scale preparation of the target compound. All the compounds were characterized by NMR and HRMS spectra.
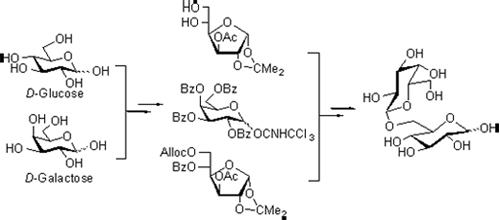
Key words: allolactose; D-glucose; D-galactose; synthesis
[1] Jobe, A.; Bourgeois, S. J. Mol. Biol. 1972, 69, 397.
[2] Jacob, F.; Monod, J. J. Mol. Biol. 1961, 3, 318.
[3] Huber, R. E.; Wallenfels, K.; Kurz, G. Can. J. Biochem. 1975, 53, 1035.
[4] Prosperi, D.; Panza, L.; Haltrich, D.; Nonini, M.; Riva, S. J. Carbohydr. Chem. 2003, 22, 267.
[5] Pazur, J. H.; Tipton, C. L.; Budovich, T.; Marsh, J. M. J. Am. Chem. Soc. 1958, 80, 119.
[6] Magnusson, G.; Nilsson, U. J. Glycoscience 2001, 2, 1543.
[7] Kong, F. Curr. Org. Chem. 2003, 7, 841.
[8] Malik, S.; Sharma, A.; Kartha, K. P. R. Trends Carbohydr. Res. 2009, 1, 36.
[9] Ma, Z.; Zhang, J.; Kong, F. Carbohydr. Res. 2004, 339, 1761.
[10] Zhang, J.; Liang, X.; Wang, D.; Kong, F. Carbohydr. Res. 2007, 342, 797.
[11] Deulofeu, V.; Deferrari, J. O. J. Org. Chem. 1952, 17, 1097.
[12] Barker, G. R. Methods Carbohydr. Chem. 1963, 2, 168.
[13] Schmidt, R. R. Angew. Chem. 1986, 98, 213.
[14] [Zhang, J.; Kong, F. Tetrahedron 2003, 59, 1429.
[15] Zong, G.; Yan, S.; Liang, X.; Wang, D.; Zhang, J. Chin. J. Org. Chem. 2011, 31, 2126 (in Chinese).
(宗广辉, 颜世强, 梁晓梅, 王道全, 张建军, 有机化学, 2011, 31, 2126.)
[16] Zong, G. H.; Yan, S. Q.; Liang, X. M.; Zhang, J. J.; Wang, D. Q.; Kong, F. Z. Chin. Chem. Lett. 2009, 20, 127.
[17] Cai, X.; Zong, G.; Xu, Y.; Zhang, J.; Liang, X.; Wang, D. Carbohydr. Res. 2010, 345, 1230.
[18] Zong, G.; Feng, Y.; Liang, X.; Chen, L.; Zhang, J.; Wang, D. Carbohydr. Res. 2010, 345, 2067.
[19] Zong, G.; Yu, N.; Xu, Y.; Zhang, J.; Wang, D.; Liang, X. Synthesis 2010, 1666.
[20] Zong, G.; Cai, X.; Liang, X.; Zhang, J.; Wang, D. Carbohydr. Res. 2011, 346, 2533.
[21] Suyama, K.; Adachi, S.; Toba, T.; Sohma, T.; Hwang, C.; Itoh, T. Agric. Biol. Chem. 1986, 50, 2069.
[22] Rio, S.; Beau, J. M.; Jacquinet, J. C. Carbohydr. Res. 1991, 219, 71.
[23] Zhang, L.; Zhang, W.; Feng, Y.;Yu, X.; Meng, X. Chem. Ind. Eng. 2004, 21, 414 (in Chinese).
(张利梅; 张卫红; 冯亚青; 于晓佳; 孟祥启, 化学工业与工程, 2004, 21, 414.)
[24] Tsui, H.-C.; Paquette, L. A. J. Org. Chem. 1998, 63, 9968.
/
| 〈 |
|
〉 |