

IPrAuCl/AgOTf催化下合成N-磺酰基四氢吡咯类化合物
收稿日期: 2013-12-19
修回日期: 2014-01-21
网络出版日期: 2014-02-14
基金资助
浙江省自然科学基金(No. Y4100558)资助项目.
Synthesis of N-Sulfonyl Pyrrolidines Catalyzed by IPrAuCl/AgOTf
Received date: 2013-12-19
Revised date: 2014-01-21
Online published: 2014-02-14
Supported by
Project supported by the Natural Science Foundation of Zhejiang Province (No. Y4100558).
原东鹏 , 曾明 , 柏春美 , 崔冬梅 , 张辰 . IPrAuCl/AgOTf催化下合成N-磺酰基四氢吡咯类化合物[J]. 有机化学, 2014 , 34(6) : 1231 -1234 . DOI: 10.6023/cjoc201312025
6-Bromofuro[2,3-d]pyrimidine bicyclic nucleosides are the key intermediates for the preparation of novel furo[2,3-d]pyrimidine bicyclic nucleoside derivatives with remarkable antiviral potency. The literature protocol for their synthesis involves a multi-step procedure and palladium catalyst. In this paper, an economical and practical method was developed for their preparation via condensation of the easily obtainable 5-formyl pyrimidine nucleosides with carbon tetrabromide and subsequent cyclization promoted by copper iodide.
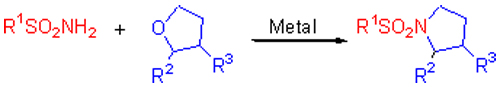
Key words: sulfonamide; catalyst; tetrahydrofuran; pyrrolidine
[1] Jain, P.; Saravanan, C.; Singh, S. K. Eur. J. Med. Chem. 2013, 60, 89.
[2] Souers, A. J.; Wodka, D.; Gao, J.; Lewis, J. C.; Vasudevan, A.; Brodjian, S.; Dayton, B.; Ogiela, C. A.; Fry, D.; Hernandez, L. E.; Marsh, K. C.; Collins, C. A.; Kym, P. R. Bioorg. Med. Chem. Lett. 2004, 14, 4883.
[3] Saku, O.; Ohta, K.; Arai, E.; Nomoto, Y.; Miura, H.; Nakamura, H.; Fuse, E.; Nakasato, Y. Bioorg. Med. Chem. Lett. 2008, 18, 1053.
[4] Podichetty, A. K.; Wagner, S.; Schröer, S.; Faust, A.; Schäfers, M.; Schober, O.; Kopka, K. Haufe, G. J. Med. Chem. 2009, 52, 3484.
[5] (a) De Boer, T. J.; Backer, H. J. Org. Synth. 1954, 34, 96.
(b) Yasuhara, A.; Kameda, M.; Sakamoto, T. Chem. Pharm. Bull. 1999, 47, 809.
(c) Kamal, A.; Reddy, S. J.; Bharathi, E. V.; Dastagiri, D. Tetrahedron Lett. 2008, 49, 348.
[6] Kim, S. K.; Gross, D. E.; Cho, D. G.; Lynch, V. M.; Sessler, J. L. J. Org. Chem. 2011, 76, 1005.
[7] Borén, L.; Leijondahl, K.; Bäckvall, J.E. Tetrahedron Lett. 2009, 50, 3237.
[8] Ruano, J. L.; Parra, A.; Marzo, L.; Yuste, F.; Mastranzo, V. M. Tetrahedron 2011, 67, 2905.
[9] Cui, D. M.; Zheng, J. Z.; Yang, L.Y. Zhang, C. Synlett 2010, 809.
[10] (a) Chen, J. Y.; Dang, L.; Li, Q.; Ye, Y.; Fu, S. M.; Zeng, W. Synlett 2012, 23, 595.
(b) Dang, L.; Li, Q.; Ma, T. M.; Sheng, S.; Zeng, W. Int. J. Org. Chem. 2011, 1, 176.
[11] Omar, W. A. E.; Heiskanen, J. P.; Hormi, O. E. O. J. Heterocycl. Chem. 2008, 45, 593.
[12] Tang, X. D.; Huang, L. B.; Qi, C. R.; Wu, X.; Wu, W. Q.; Jiang, H. F. Chem. Commun. 2013, 49, 6102.
[13] Heinrich, D. M.; Flanagan, J. U.; Jamieson, S. M. F.; Silva, S.; Rigoreau, L. J. M.; Trivier, E.; Raynham, T.; Turnbull, A.P.; Denny, W. A. Eur. J. Med. Chem. 2013, 62, 738.
[14] Zhou, G.; Ting, P.; Aslanian, R.; Piwinski, J. J. Org. Lett. 2008, 10, 2517.
[15] Yin, Y.; Zhao, G. J. Fluorine Chem. 2007, 128, 40.
[16] Yin, G. Y.; Wu, T.; Liu, G. S. Chem. Eur. J. 2012, 18, 451.
[17] Zhang, G. Z.; Cui, L.; Wang, Y. Z.; Zhang, L. M. J. Am. Chem. Soc. 2010, 132, 1474.
/
| 〈 |
|
〉 |