

红树尖瓣海莲内生真菌Penicillium sp. J41221的代谢产物及其抗菌活性研究
收稿日期: 2014-01-09
修回日期: 2014-01-26
网络出版日期: 2014-02-14
基金资助
海南省自然科学基金(No. 213021)、国家自然科学基金(Nos. 81160391,81360478,21162009)、海南省大学生创新训练(No. 2013116580)资助项目.
Secondary Metabolites and Antibacterial Activities of a Bruguiera sexangula var. Rhynchopetala-Derived Fungus Penicillium sp. (J41221)
Received date: 2014-01-09
Revised date: 2014-01-26
Online published: 2014-02-14
Supported by
Project supported by the Natural Science Foundation of Hainan Province (No. 213021), the National Natural Science Foundation of China (Nos. 81160391, 81360478, 21162009) and the National Undergraduate Innovation Training Programs (No. 2013116580).
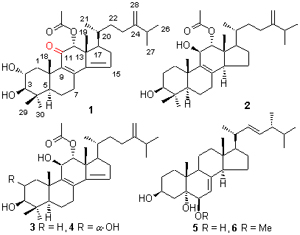
在生物活性指导下,从一株来源于药用红树尖瓣海莲的内生真菌Penicillium sp.(J41221)中分离鉴定了6个化合物,包括4个四环三萜类化合物和2个甾醇类化合物,结构分别为:11-羰基-12α-乙酰氧基-4,4-二甲基-24-甲烯基-5α-胆甾-8,14-二烯-2α,3β-二醇(1),12α-乙酰氧基-4,4-二甲基-24-甲烯基-5α-胆甾-8-单烯-3β,11β-二醇(2),12α-乙酰氧基-4,4-二甲基-24-甲烯基-5α-胆甾-8,14-二烯-3β,11β-二醇(3),12α-乙酰氧基-4,4-二甲基-24-甲烯基-5α-胆甾-8,14-二烯-2α,3β,11β-三醇(4),啤酒甾醇(5)和(3β,5α,6β,22E)-6-甲氧基麦角甾-7,22-二烯-3,5-二醇(6). 其中化合物1为首次从生物中获得,且1和2的波谱数据迄今未见任何报道. 抗菌活性结果表明,化合物2和4对金黄色葡萄球菌、大肠杆菌和四联球菌均显示一定的抑制活性,最小抑菌浓度(MIC)分别为5和4.86 μmol/L.
关键词: 尖瓣海莲; Penicillium sp.; 次级代谢产物; 三萜类化合物; 抗菌活性
郑彩娟 , 黄国雷 , 唐雄肇 , 王德能 , 龚小路 , 张强 , 宋小平 , 陈光英 . 红树尖瓣海莲内生真菌Penicillium sp. J41221的代谢产物及其抗菌活性研究[J]. 有机化学, 2014 , 34(6) : 1172 -1176 . DOI: 10.6023/cjoc201401014
Under the guidance of bioassay, four tetracyclic triterpenoids and two steroids were isolated from the fermentation products of Penicillium sp. (J41221), a fungus obtained from a mangrove Bruguiera sexangula var. Rhynchopetala. Their stru- ctures were identified as 11-oxo-12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8,14-diene-2α,3β-diol (1), 12α- acetoxy- 4,4-dimethyl-24-methylene-5α-cholesta-8-momoene-3β,11β-diol (2), 12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta- 8,14-diene-3β,11β-diol (3), 12α-acetoxy-4,4-dimethyl-24-methylene-5α-cholesta-8,14-diene-2α,3β,11β-triol (4), cerevisterol (5) and (3β,5α,6β,22E)-6-methoxyergosta-7,22-diene-3,5-diol (6). Among them, compound 1 was a new naturally occurring compound, and 1 and 2 have no spectroscopic data reported until now. Compounds 2 and 4 showed inhibitory activities against Staphylococcus aureus, Escherichia coli and Micrococcus tetragenu, with minimum inhibitory concentration (MIC) values of 5 and 4.86 μmol/L, respectively.

[1] Blunt, J. W.; Copp, B. R.; Keyzers, R. A.; Munro, M. H. G.; Prinsep, M. R. Nat. Prod. Rep. 2013, 30, 237.
[2] Lin, P. Mangrove, Ocean Press, Beijing, 1984, p. 96 (in Chinese).
(林鹏, 红树林, 海洋出版社, 北京, 1984, p. 96.)
[3] Wang, Y.-S.; He, L.; Wang, Q.-J.; Zhang, S. Chin. J. Mar. Drugs 2004, 24, 26 (in Chinese).
(王友绍, 何磊, 王清吉, 张偲, 中国海洋药物, 2004, 24, 26.)
[4] Shao, C.-L.; Fu, X.-M.; Wang, C.-Y.; Han, L.; Liu, X.; Fang, Y.-C.; Liu, G.-X.; Guan, H.-S. J. Ocean Univ. China 2009, 39, 712 (in Chinese).
(邵长伦, 傅秀梅, 王长云, 韩磊, 刘新, 方玉春, 李国强, 刘光兴, 管华诗, 中国海洋大学学报, 2009, 39, 712.)
[5] Singh, S. B.; Zink, D.; Dombrowski, A. W.; Polishook, J. D.; Ondeyka, J. G.; Hirshfield, J.; Felock, P.; Hazuda, D. J. Bioorg. Med. Chem. 2003, 11, 1577.
[6] Singh, S. B. Tetrahedron Lett. 2000, 41, 6973.
[7] Yagen, B.; Horn, P.; Joffe, A. Z.; Cox, R. H. J. Chem. Soc., Perkin Trans. 1 1980, 2914.
[8] Ashe, B. M.; Fletcher, D. S. US 4874755, 1989 [Chem. Abstr. 1989, 4872727].
[9] Kawagishi, H.; Katsumi, R.; Sazawa, T.; Mizuno, T.; Hagiwara, T.; Nakamura, T. Phytochemistry 1988, 27, 2777.
[10] Ling, S. K.; Komorita, A.; Tanaka, T.; Fujioka, T.; Mihashi, K.; Kouno, I. J. Nat. Prod. 2002, 65, 656.
[11] Xiang, Z.-J.; Zhao, G.-R.; Yuan, J.-J.; Guo, Z.-X. Chin. Trad. Herb. Drugs 2006, 37, 211 (in Chinese). (向志军, 赵广荣, 元英进, 郭治昕, 中草药, 2006, 37, 211.)
[12] Solis, P. N.; Wright, C. W.; Anderson, M. M.; Gupta, M. P.; Phillipson, J. D. Planta Med. 1993, 59, 250.
[13] Meyer, B. N.; Ferrigni, N. R.; Putnam, J. E.; Jacobsen, L. B.; Nichols, D. E.; McLaughlin, J. L. Planta Med. 1982, 45, 31.
/
| 〈 |
|
〉 |